Ozonolysis is an organic chemical reaction in which ozone is used to cleave the unsaturated bonds of alkenes, alkynes, and azo compounds. It is a reaction in which ozone is used to break the unsaturated bonds of alkenes, alkynes, and azo compounds (compounds with the functional diazenyl functional group). It is a redox reaction in the organic realm. The oxidation of alkenes with the assistance of ozone can result in the formation of alcohols, aldehydes, ketones, and carboxylic acids. When alkynes are subjected to ozonolysis, they yield acid anhydrides or diketones. If there is any water present in the process, the acid anhydride will undergo hydrolysis, resulting in the formation of two carboxylic acids. Ozonolysis of elastomers is also referred to as ozone cracking in some circles. The presence of trace amounts of ozone gas in the atmosphere causes double bonds in elastomers to be broken. The ozonolysis of azo compounds results in the formation of nitrosamines.
Ozonolysis of Alkenes
Alkenes can be converted to alcohols, aldehydes, ketones, and carboxylic acids by the process of ozonolysis. The general approach calls for the use of an alkene solution in methanol. At a temperature of around 780 degrees Celsius, ozone bubbles through this solution. When the solution turns blue, this indicates that the alkene has been consumed (the blue colour comes from the unreacted ozone). Among the other markers of the reaction’s completion is the presence of potassium iodide solution. To make this indicator, a stream of oxygen that has been treated with ozone is bubbled through a solution of the reactants. During the process, the gas that bubbles up is routed via an iodide solution of potassium. Once the reaction is complete and there is no longer any alkene available to react with the ozone, the gas continues on to oxidise potassium iodide to iodine in the presence of oxygen. The formation of iodine as a result of the reaction of potassium iodide with ozone is evidenced by the distinctive violet colour of iodine.
The addition of ozone to the reaction mixture necessitates the addition of a reagent in order to transform the ozonide into the necessary carbonyl derivative. There are two strategies that can be used for this conversion:
- Reductive Workup
- Oxidative Workup
When compared to oxidative workup circumstances, reductive workup conditions are utilised significantly more frequently. Triphenylphosphine, thiourea, zinc dust, and dimethyl sulphide are all substances that can be employed to create aldehydes or ketones under these working circumstances. The usage of hydrogen peroxide, on the other hand, can be utilised to create carboxylic acids. Ozone can also be used to oxidise other functional groups, such as benzyl ethers, which are a type of organic compound. Due to the possibility of minor amounts of acid being formed during the reaction, pyridine is also added to buffer the reaction. A 1:1 mixture of solvent and dichloromethane co-solvent makes it easier to get the ozonide to cleave in a timely manner. Agriculturally, the process of ozonolysis of oleic acid is utilised to manufacture azelaic and pelargonic acids on an industrial scale.
The Mechanism of Ozonolysis of Alkenes
Step 1
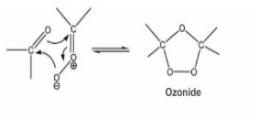
The electrophilic addition of ozone to the carbon-carbon bond results in the formation of the molozonide intermediate, which is a highly unstable intermediate state. As a result of its unstable nature, the molozonide continues to react, breaking apart to generate a carbonyl molecule and a carbonyl oxide molecule, as seen in the illustration below:
Step 2
During step 1, the carbonyl molecule and the carbonyl oxide molecule that were formed rearrange themselves, reforming to form an intermediate that is more stable than carbonyl oxide. This ozonide intermediate can be exposed to either an oxidative or a reductive workup, depending on the situation. Carboxylic acid will be the product of the oxidative workup, whereas aldehydes or ketones will be the product of the reductive workup.
Following is a diagram depicting the production of the ozonide intermediate.
Oxidative Workup
When the oxidant hydrogen peroxide is employed to treat the ozonide in place of zinc or dimethyl sulphide, the aldehydes generated are oxidised to carboxylic acids, which is the desired result. It is also possible to utilise potassium permanganate in the presence of hot acid to aid in the oxidative workup.
Reductive Workup
A weak reducing agent, such as dimethyl sulphide or zinc metal, is used in conjunction with water to treat ozonide in this process. The ozonide is decreased in the manner depicted below.
Ozonolysis of Alkynes
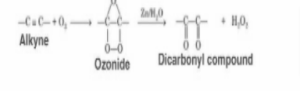
Alkynes are subjected to ozonolysis, which results in the formation of an acid anhydride or a diketone as the final product. In this reaction, the fragmentation is not completely completed (alkenes undergo complete fragmentation). Because of the simple aqueous workup that is used, no reducing agents are required. It is possible that the acid anhydride will undergo hydrolysis, resulting in the formation of two carboxylic acids if the reaction takes place in the presence of water. When determining the position of the triple bond in an unknown alkyne, the technique of Ozonolysis can be used.
Ozonolysis of Alkynes Mechanism
The reaction between the alkyne and the ozone results in the breakdown of the alkyne. This results in the formation of the ozonide intermediate. With the assistance of zinc metal, a straightforward aqueous reaction is carried out, ultimately resulting in the formation of a dicarbonyl molecule. The following is an example of how to express the reaction:
CONCLUSION
It is possible to determine the location of double or triple bonds in alkenes and alkynes using the technique of ozonolysis, which is employed in organic chemistry. Ozonolysis is a technique that is used to determine the structure of lengthy alkenes and alkynes. Ozonolysis is a process that is utilised in bleaching. Ozonolysis is a technique for disinfecting wastewater. Ozonolysis is a chemical reaction that is employed in the production of alcohols, carboxylic acids, aldehydes, and ketones.
The mechanism of ozonolysis is composed of three phases that involve reactions. 1- The ozone molecule comes under attack. Forming of the ozonide intermediate in step 2. Carbonyl compounds are formed in step three.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out