Ozonolysis:
Ozonolysis is a redox chemical reaction where unsaturated carbon having either double or triple bonds of alkenes (CnH2n), alkynes (CnH2n-2) or azo compounds are cleaved with ozone.
Oxidation of alkenes with the help of ozone yields alcohol (-OH), aldehydes (-CHO), ketones (-C=O), or carboxylic acids (-COOH).
Oxidation of alkynes results in anhydrides or diketones. If in the chemical reaction, H2O molecule is present then the anhydrides would undergo hydrolysis giving 2 carboxylic acids.
Oxidation of elastomers is also called ozone tracking. This reaction occurs if trace amounts of ozone is present in the atmosphere which cleave the double bond of elastomers.
Ozone (O3): Ozone is an allotrope of oxygen (O2) molecules. Ozone is an inorganic molecule and has 3 oxygen molecules in its structure called trioxygen. In terms of stability, ozone is less stable than oxygen molecules. Bent structure of ozone has a polar molecule. Central atom of the ozone molecule has less e- density due to which there is uneven distribution of electrons across the atoms resulting in the polarity of molecules. Ozone has 2 resonating structures. The charge distribution reveals that the central atom has a charge of +1 and other 2 oxygen atoms have -½ charge on each atom.
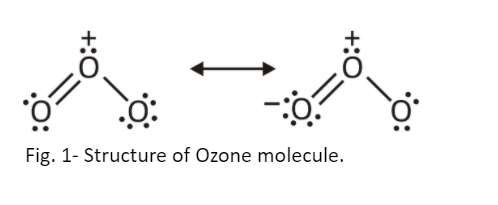
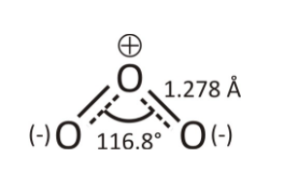
Fig. 2- Resonating structure of Ozone (O3).
Mechanism of Ozonolysis:
Mechanism of ozonolysis is mainly based on oxidative cleavage reaction. Ozone molecule breaks the pi bond and carbon-carbon sigma bond in the cleavage reaction during the process. Ozone attack on the reactant forms ozonide. During the reaction, when the intermediate stage is reached zinc (Zn) dust is employed to remove the oxygen so to avoid formation of zinc oxide in presence of oxygen. End product is obtained according to the type of reactant present in the reaction mixture.
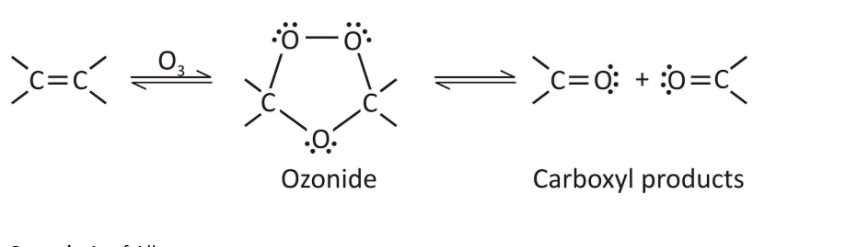
Fig. 3- Ozonolysis reaction.
Ozonolysis of Alkenes:
Mechanism of Ozonolysis of Alkenes is a 2-step process.
Step 1- Oxidation of Alkene or Molozonide and ozonide formation:
Molozonide acts as an intermediate in the chemical reaction which is a 5-membered ring structure formed as when ozone reacts with the double bond of alkene. Molozonide then rearranges to form ozonide.
Fig. 4- Formation of molozonide in ozonolysis of alkenes.
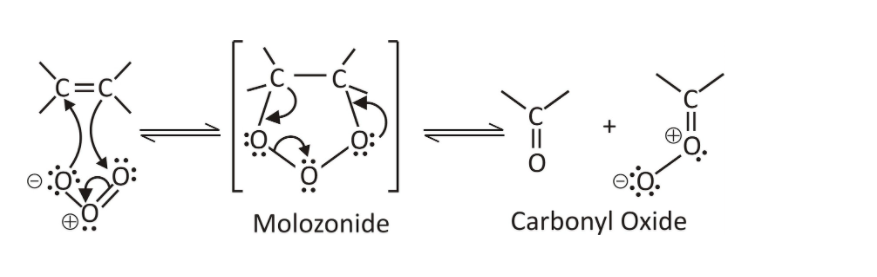
Step 2- Ozonide reduction:
With the help of mild reagents such as zinc or dimethyl sulphide, ozonide can be reduced to aldehydes or ketones. When dimethyl sulphide (acts as reducing agent) is used sulphur gets oxidized and forms dimethyl sulfoxide also called as DMSO.
Fig. 5- Formation of aldehydes or ketones as the end product of the chemical reaction.

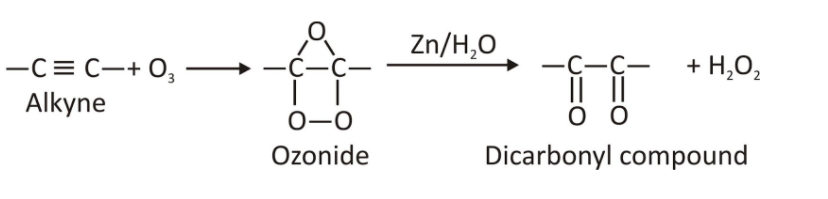
Ozonolysis of Alkynes:
The exact mechanism of ozonolysis is not completely known. Here in this mechanism of ozonolysis of alkynes there is no use of reducing agent. The mechanism can be explained in the 2-step process.
Step 1- Ozonide formation:
Triple bond of alkyne breaks on reaction with the ozone resulting in the formation of a cyclic compound called as ozonide.
Step 2- Formation of diketones and carboxylic acids:
Ozonide reduction yields diketone compounds which upon hydrolysis gives carboxylic acids.
Fig. 7- Mechanism of ozonolysis of alkyne
Ozonolysis of Elastomers:
Trace amount of ozone present in the atmosphere creates cracks in the elastomers (rubber like solids having elastic properties). This is because ozone attacks the double bond in the elastomers.
Under tension, cracks begin to form and appear around the circumference of a rubber tube as it is inclined at 90o to the axis of the strain. In everyday life, these cracks appear to be harmful as these cracks may occur in fuel pipes exposing the exterior surface which may lead to a case of leakage and fires.
Applications of Ozonolysis:
- In organic chemistry, ozonolysis is used to locate double or triple bonds in alkenes and alkynes respectively.
- To determine the structure of alkenes or alkynes.
- Ozonolysis is used in bleaching.
- Its application can be seen in wastewater disinfection.
- Its applications are deployed for commercial purposes as well. It is used in the synthesis of alcohols, carboxylic acids, aldehydes, and ketones.
Polymerization:
Our daily life has become easier and colorful with the discovery and varied applications of polymers. The use of polymers in the manufacture of plastic buckets, cups, toys, synthetic clothing materials, etc. has revolutionized daily life as well as the industrial sector. Polymers are the backbone for industries like plastics, elastomers, fibres and paints and varnishes.
The word ‘polymer’ has been coined from the Greek word- poly meaning many and mer meaning until or part. Polymer can be defined as a large number of molecules having molecular mass ranging from 103-107u. These are also referred to as macromolecules formed by addition of repeating structural units called monomers linked each other by covalent bonds. This process of formation of polymers is called polymerization.
Classification of Polymer:
On the basis of source from polymer is derived, it can be categorized into following types-
- Natural Polymers: These polymers are found in plants and animals.
For e.g.- cellulose, proteins, starch, resins and rubber.
- Semi-synthetic Polymers: Cellulose derivatives like cellulose acetate and cellulose nitrate fall in this subcategory.
- Synthetic Polymers: Polymers such as plastic (polythene), synthetic fibres (nylon 6,6) and synthetic rubber (Buna-S) are examples of man-made polymers.
On the basis of structure of polymers, it is categorized into following types-
- Linear polymers: Comprises long and straight-chain of monomers.
- Branched polymers: These are linear polymers consisting of branches.
- Cross linked polymer: Constitute cross linked polymers linked with each other. These are bi-functional or tri-functional monomers.
On the basis of forces between molecules, polymer can be categorized into following types-
- Elastomers- These are rubber like solids having elastic properties. These are held together by weak intermolecular forces allowing polymers to stretch.
E.g.- Buna-S
- Fibers- They have strong intermolecular forces like hydrogen bonding. Molecules are closer to each other and are crystalline in nature.
E.g.- Polyamide and Polyesters
- Thermoplastic polymers- Consists of linear or branched polymer that can be softened on heating and hardened on cooling.
E.g.- Polyvinyl, polystyrene
- Thermosetting polymers- Polymers are heavily branched and cross linked with each other. These polymers cannot be reused.
For e.g.- Bakelite
Types of Polymerizations:
- Condensation Polymerization: When two different bifunctional or tri-functional monomer units react, they form a condensation polymer.
E.g., Silicon, Bakelite, Nylon, etc.
- Addition Polymerization: In this polymerization process, double or triple bonded monomers are repeatedly added to form a polymer. No by-products are formed in this reaction.
For e.g.- Propene (C3H6) helps in the formation of poly-propene ((C3H6)n).
This is further classified into 2 categories-
- Copolymers- Polymers formed due to the addition reaction between two different kinds of monomers are called as copolymers.
For e.g.- Buna-N and Buna-S
- Homopolymers- Polymers formed due to addition reaction between same kinds of monomers are called as homopolymers.
For e.g.- Polythene
Mechanism of polymerisation: The mechanism of polymerization mainly is a 3-step process that includes- initiation, propagation and termination.
The mechanism of polymerization involves 2 methods-
- Step-growth Polymerization
- Chain growth Polymerization
Step-growth Polymerization: In this process of polymerization, monomers units with functional groups independently combine to form polymer. In this process two polymer of different or same length forms a longer molecule. It is a time taking and lengthy process.
E.g., Condensation polymerisation – In this process, H2O molecule evolved in the reaction lengthen the chain. Polyamides, polyesters, phenol-formaldehyde and melamine-formaldehyde are examples of condensed polymerization.
Chain growth Polymerization: In this polymerization, monomers add together to form a long chain. These monomers added may be of same type or different. Alkenes, alkynes and their derivatives are used in this process of polymerization. Lengthening of chain occurs as a result of either free radicals or ionic species.
Free Radical Mechanism:
This mechanism follows a 3-step process that includes-
- Chain-Initiation step
- Chain-Propagating step
- Chain-Terminating step
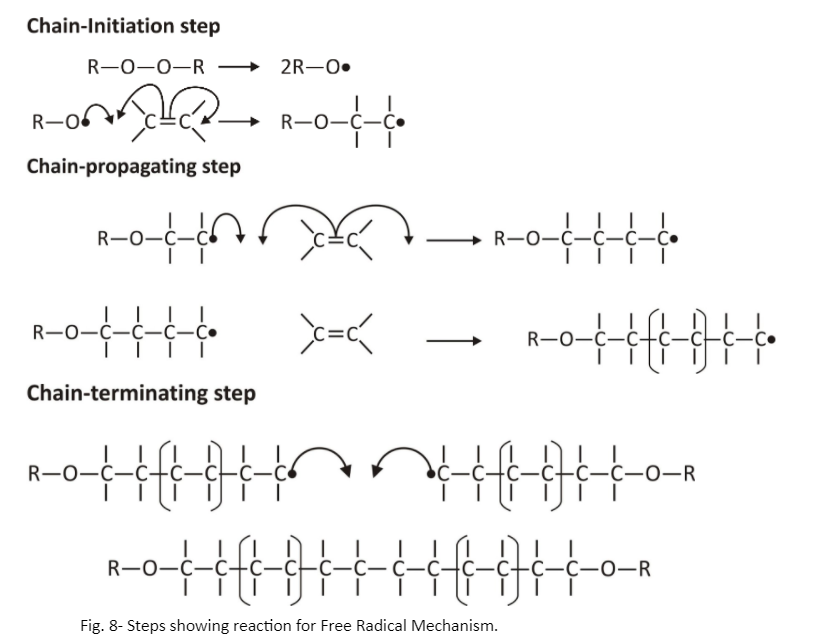
Fig. 8- Steps showing reaction for Free Radical Mechanism.
Preparation of polymers:
- Low-Density Polythene- This type of polymerization is obtained under high pressure of 1000-2000 atm and at temperature of 350-520 K in the presence of dioxygen or peroxide as a catalyst.
- High-Density Polythene- In the presence of catalyst like triethyl aluminium and titanium tetrachloride, polymerization addition of ethene takes place. A condition of low pressure of 3-4 atm and 343K temperature is required.
Conclusion
The conditions favorable for ozonolysis is O3+ alkene and the end product obtained is in the form of carbonyl compound either an aldehyde, ketone, or carboxylic acid (if H2O2). Ozonolysis application has wide aspects in our everyday life and in commercial purposes as well. Similarly, the polymers play an immense role in beautifying our life with colorful objects like toys, plastic materials,etc.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




