Introduction:
Are you preparing for the prestigious IIT-JEE exam this year? Unacademy brings the best topic-wise notes on all important subjects including chemistry for IIT-JEE aspirants. Out of all important topics, let us discuss the group 17 elements of the periodic table in this section, i.e., about halogens. It is derived from the Greek words, “Hal” for salt and “Gen” for reproduction. These are fluorine (F), chlorine (Cl), bromine (Br), iodine (I), tennessine (Ts), and astatine (At). These are highly reactive elements and hence are not found in nature.
If you’re looking to prepare for the IIT-JEE examination, below is the short and crisp material by Unacademy on the properties, structure, and types of these oxides and oxyacids of halogens.
What are the oxides of halogens?
All halogens form multiple oxides due to the covalent nature of halogen and oxygen. While most halogen oxides are unstable, there is a large similarity between the electronegativity of halogens and oxygen. As fluorine is more electronegative than oxygen, hence all compounds of fluorine with oxygen are termed fluorides of oxygen instead of termed as oxides of fluorine.
Fluorine is one of the most abundant halogens that are present in the earth’s crust in combined form. All these elements show great resemblance to one another in terms of chemical and physical properties. Their oxides and oxoacids are formed widely but only a few of them are known due to their reactive nature.
When it comes to stability, I2O5 is the only oxide of halogen that remains stable with respect to the dissociation of the elements. The oxides of bromine are the least stable while chlorine oxides decompose violently.
The top use of halogens includes the use of Cl20 and ClO2 as bleaching agents in the pulp and flour industries. When it comes to employing the I2O5, it is achieved in the estimation of CO. Some of the famous oxides of halogens include:
- Fluorides: These are in the form of OF2(-1 oxidation state) and O2F2 (-1 oxidation state).
- Chlorine oxides: These are Cl2O (+1 oxidation state), ClO2(+4 oxidation state), Cl2O6(+6 oxidation state), and Cl2O7 (+7 oxidation state).
- Bromine oxides: These are Br2O (+1 oxidation state), BrO2(+4 oxidation state), BrO3 (+6 oxidation state).
- Iodine oxide: I2O5 (+5 oxidation state).
Structure:
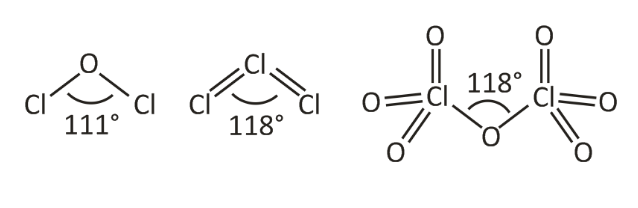
Out of all the halogen oxides, a few of their structures are known. The monoxide structures can be explained based on VSEPR theory. These have a tetrahedral structure with two lone oxygen pairs that give the molecule an angular and “V” shape. The value of bond angle differs based on the position of electrons.
F-O-F< Cl-O-Cl< Br-O-Br
The bonded electron pairs are closer to oxygen in Br2O and Cl2O that makes them repel each other and reduces the lone pair-lone pair repulsion on oxygen.
Properties:
The halogen elements form unstable oxides as they encompass free positive energies of formation. OF2 is the exception as it is stable up to 475 Kelvin with respect to the dissociation of the elements. The stability of these oxides decreases in the below range based on the kinetic and thermodynamic factors:
I > Cl > Br
All the higher oxides are stable compared to the lower ones and all oxides except that of iodine are explosive. Iodine pentoxide I2O5 is in the form of a white solid that is stable up to 575 Kelvin. Cl2O and ClO2 are further used as germicides and bleaching agents.
What are the oxoacids of halogens?
Any acid containing hydrogen, oxygen, and any other element is called oxoacid. If oxygen is present in acid, the suffixes “ous” and “ic” are used to represent the lower and higher number of oxygen atoms in the acid formula.
Group 17 of the periodic table- fluorine, chlorine, bromine, iodine, and astatine are the salt producers. A regular gradation in the physical and chemical properties of all elements is observed while moving from up to the bottom.
While all these elements are highly related, Astatine is the only radioactive element in the group. The electronic configuration of these elements indicates that they have seven electrons in the valence shell with ns2np5 configuration. Hence, instead of losing electrons, these elements gain one electron to achieve stable configurations. Due to the small size and effective nuclear charge, the different oxoacids formed by these elements include:
- Hypohalous acid: It is HOX and has a +1 oxidation state. Some of its examples are HOF, HOCl, HOBr, HOI, etc.
- Halous acid: It is HXO2 and has a +3 oxidation state. Some of its examples are HClO2, etc.
- Halic acid: It is HXO3 and has a +5 oxidation state. Some of its examples are HClO3, HBrO3, HIO3, etc.
- Perhalic acid: It is HXO4 and has a +7 oxidation state. Some of its examples are HClO4, HBrO4, HIO4, etc.
Structure:
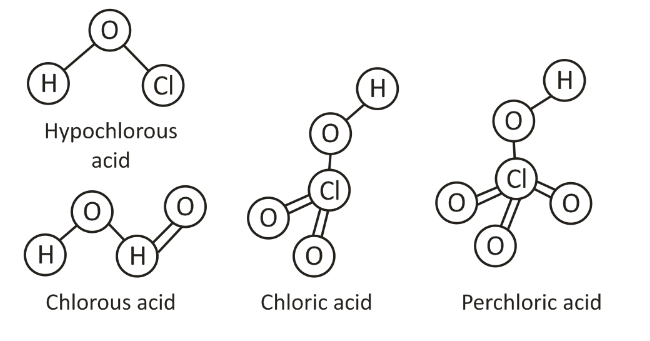
The common structure of halogen group oxoacids reveals that their central atom is sp3 hybridized. One X-OH bond is compulsorily present in every oxoacid. The other commonly present bonds in oxoacids are X= O. It is a double bond between hydrogen and oxygen that is dpi-dpi in nature.
Due to the high electronegativity and small size of the atom, the first member of the group has the highest acidic strength. This acidic strength further increases with an increase in the oxidation number of these elements.
Properties:
All members of group 17 periodic table form a variety of oxoacids. The only exception is fluorine that forms only one oxoacid, i.e. hypofluorous acid or fluoric (I) acid. It is denoted by HOF. All other elements can’t stay isolated in the pure state. All these elements are highly stable in the form of salts or in aqueous solutions.
Going by the structure, it can form four series of oxoacids, i.e, hypohalous
acids (+1 oxidation state), halous acids (+3 oxidation state), halic acids (+5 oxidation state), and perhalic acid (+7 oxidation state).
The popular oxoacids of halogens include the oxoacids of chlorine and bromine. The chlorine forms four oxoacids namely hypochlorous acid (HOCl), chlorous acid (HOClO), chloric acid (HOClO2), and perchloric acid (HOClO3). The bromine forms three oxoacids namely hypobromous acid (HOBr), bromic acid (HOBrO2), and perbromic acid (HOBrO3). The iodine forms three oxoacids namely hypoiodous acid (HOI), iodic acid (HOIO2), and periodic acid (HOIO3).
Conclusion:
Oxides and oxoacids of halogens form an interesting part of the IIT-JEE chemistry syllabus. There are four series of oxoacids namely hypocephalus, halous acids, halic acids, and perhalic acid. The oxides include fluorides, chlorine oxides, bromine oxides, iodine oxides, etc. All these oxides and oxoacids have different properties based on their structure and are used accordingly in the chemical sciences.
It is important to know about the arrangement of halogens in the periodic table, their electronegativity, and their stability. The acidic strength and electronic configuration help determine the reactivity and thus the stability of these elements. There are certain interhalogen compounds that are formed with the reaction of the two halogens only.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





