Known in organic chemistry as the Wolff–Kishner reduction, this reaction is used in the conversion of carbonyl functionalities into methylene groups. A carbonyl group is most frequently removed after serving its synthetic purpose of activating an intermediate in a preceding step in the context of complex molecule synthesis, which is the most common application of this technique. The consequence of this reaction is that there is no obvious retron for it. It was Nikolai Kischner in 1911 who first reported on the reaction, followed by Ludwig Wolff in 1912.
Mechanism
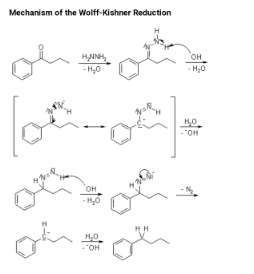
Szmant and colleagues have conducted research into the mechanism of the Wolff–Kishner reduction in detail. In accordance with Szmant’s research, the first step in this reaction is the formation of an anion 1 hydrazone by deprotonation of the terminal nitrogen by MOH, which is followed by the formation of an anion 2 hydrazone. When semicarbazones are used as substrates, the first step is the conversion of the semicarbazone into the corresponding hydrazone, which is then followed by deprotonation. According to a variety of mechanistic data, the rate-determining step involves the formation of a new carbon–hydrogen bond at the carbon terminal of the delocalized hydrazone anion at the carbon terminal. This proton capture occurs in a coordinated manner with the abstraction of the second proton at the nitrogen terminal, which is caused by a solvent-induced abstraction. In support of this mechanistic proposal, Szmant’s discovery that this reaction is first order in both the hydroxide ion and the ketone hydrazone is noteworthy. A number of solvent molecules must be involved in this process in order for it to be considered a coordinated process. A detailed Hammett analysis] of aryl aldehydes, methyl aryl ketones, and diaryl ketones revealed a non-linear relationship, which the authors attribute to the complexity of the rate-determining step in the synthesis process. Mildly electron-withdrawing substituents promote the formation of carbon-hydrogen bonds, whereas highly electron-withdrawing substituents reduce the negative charge at the terminal nitrogen, resulting in a larger and more difficult solvation shell, which makes the breaking of the N-H bond more difficult. In the proposed transition state, there is a high degree of organisation, which can be explained by the exceptionally high negative entropy of activation values that have been observed.
Furthermore, it was discovered that the rate of the reaction is influenced by the concentration of the hydroxylic solvent as well as the cation present in the alkoxide catalyst Crown ether present in the reaction medium has the potential to increase the reactivity of the hydrazone anion 1 by dissociating the ion pair, thereby increasing the reaction rate. When a proton source is present, the diimide anion 2 collapses, releasing the hydrocarbon. This is accomplished through the loss of dinitrogen, which yields an alkyl anion 3, which then undergoes a rapid and irreversible acid-base reaction with the solvent, resulting in the formation of the alkane. Taber discovered this high-energy intermediate through the use of intramolecular trapping, which he published in Science. An alkyl anion intermediate appeared to be more consistent with the stereochemical outcome of this experiment than with the alternative possibility of an alkyl radical, according to the results. The evolution of nitrogen gas from the reaction mixture serves as the overall driving force of the reaction.
Applications:
In order to complete the total synthesis of scopadulcic acid B, aspidospermidine, and dysidiolide, the Wolff–Kishner reduction has been used successfully. The Wolff–Kishner reduction is a useful tool in organic synthesis because of its efficiency. One of the final steps in the synthesis of (±)aspidospermidine, for example, was carried out by Ishibashi and colleagues using the Huang Minlon modification of the Wolff–Kishner reduction, which was developed by Huang Minlon. After hydrazone formation at 160 °C, the distillable material was removed and the mixture was then heated to 210 °C overnight. Carbonyl groups reduced in the Wolff–Kishner reduction were required for steps in the preceding synthesis that were not achieved otherwise. It was found that the tertiary amide was stable under the reaction conditions and that it could be reduced by lithium aluminium hydride. The consequence of this reaction is that there is no obvious retron for it. It was Nikolai Kischner in 1911 who first reported on the reaction, followed by Ludwig Wolff in 1912.
As demonstrated in the preceding example, amides are typically not suitable substrates for the Wolff–Kishner reduction procedure. Coe and colleagues, on the other hand, discovered that a twisted amide can be reduced efficiently under Wolff–Kishner conditions. Because of the stereoelectronic bias present in the substrate, the authors propose that “anti–Bredt” iminium ion formation is prevented, resulting in the ejection of alcohol and the formation of hydrazones being favourably affected. Given the fact that resonance stabilisation is prevented by torsional restrictions in this strained substrate, the amide functionality can be thought of as isolated amine and ketone functionalities in this system. A two-step procedure resulted in an overall yield of 68 percent for the product in question.
Conclusion:
The Wolff–Kishner reduction reaction is a type of organic chemistry reaction that is used to convert carbonyl functionalities into methylene groups. For this reason, the Wolff–Kishner reduction is incompatible with base-sensitive substrates, as it requires extraordinarily simple conditions to be achieved. In some cases, carbonyl groups that are sterically hindered will fail to form the necessary hydrazone, preventing the reaction from taking place. This approach may be preferable to the associated Clemmensen reduction in the case of compounds containing acid-sensitive functional groups, such as pyrroles, and in the case of high-molecular-weight compounds.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





