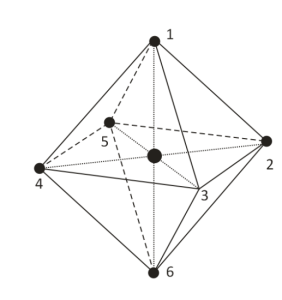
The octahedral shape of molecules is the shape of molecules where six atoms or ligands or groups of atoms are arranged in a systematic way around a central dogma or atom. The Octahedral Shape of Molecules contains eight faces. It has two square pyramids back to back, each square pyramid with four faces. That’s why this is known as octahedral. It has the prefix octa which means eight. An example of octahedral compounds is molybdenum hexacarbonyl (Mo(CO)6). The arrangement of the surrounding atom has given octahedral molecules their overall shape of eight connected triangles. All the bonds in which an octahedral shape of molecules have made a 90-degree angle with each other or in other words it have a bond angle of 90 degrees. Molecules that have octahedral structures have square planar geometry. As we all know that a perfect octahedron belongs to the point ‘Oh’. Octahedral shape of molecules has a steric number which is six.
- When more than one ligand or we can say atoms are attached to the centre of an octahedral atom, then the complex which is formed by the attachment of these atoms can also exist in isomers.
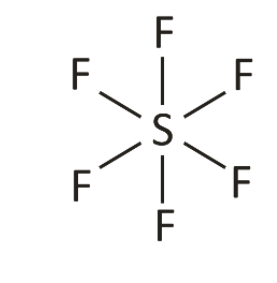
- The octahedral shape of molecules has a generic formula which is XF6. Where X is a central metal and F is the ligands attached to the central atom to form the octahedral shape. For example, sulphur hexafluoride (SF6) where S (sulphur) is the central atom and F (fluorine)is the atom or ligand attached to the central atom.
- If lone pairs are absent in the compounds then the molecular geometry matches with the geometry of electronics and is octahedral. At this point, the angle formed by the base bonds is 180° and 90°. there is no reason present to tweak the bonds to the other values.
- The octahedron shape of molecules is nonpolar in nature. As long as the six positions of the atoms are the same, because of perfect symmetry the molecule present cannot be polar in nature. Also, as long as the same or we can say identical atoms are laid opposite to each other or in trans isomers In which atoms lie 180°, even mixed peripheral atoms can still be nonpolar in nature.
- Elements that have octahedral geometry or octahedral shape of molecules are SF6, [Co(NH3)6]3-, (Mo(CO)6) etc.
Octahedral shape
It is well known that the octal prefix means eight and an octagon polygon contains eight sides and we know octuplets are a group of eight. So the question will arise that when everything starting with octa has eight sides, then why is the molecule with six bonds known as octahedral. To answer all these questions we have to look after the shape of the molecules which are formed by the outer atoms of the molecules of the octahedron. Let’s imagine that the outer atoms of the octahedron are well connected and we can see that octahedral atoms which are connected will form triangles on each side of the octahedral molecules. If we were to unfold these we will see that it has two-dimensional shapes, they would appear as eight triangles connected.
Octahedral terminology :
Octahedral is a term used by chemists, researchers by only focusing on the bonds formed by the central atom and by neglecting the difference which ligands have in themselves. Term Octahedral is mainly given because its compounds have eight faces and molecules in the compounds are joined to form octahedra.
Isomerism In octahedral complexes
When two or more types of ligands are attached to the octahedral centre then the complex can exist as isomers. Octahedral complexes can exist in two types of isomers, cis and trans.
Cis isomers :- In cis isomers ligands present are mutually adjacent to each other, they lie opposite to each other.
Trans isomers :- In trans isomers ligands present at 180 degrees to each other, they lie in front of each other.
Conclusion
The octahedral shape of molecules meaning is the molecules that contain eight faces. We study octahedral features in many chapters in our chemistry textbook and this concludes that octahedral geometry is one of the important parts of our chemistry. It contains a total of seven atoms one is the central atom and the other six are side atoms that join with a central atom in a manner to form octahedral compounds. When any lone pair comes then the octahedral shape is called distorted octahedral structure because as we all know that lone pair -lone pair repulsion is stronger than the repulsion between lone pair-bond pair.
That’s why octahedral compounds do not contain any lone pair.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out