When an electron-pair donor reacts with an electron pair acceptor, the reaction is termed a nucleophilic substitution reaction. The electron-pair donor is termed a nucleophile.
They are named so because they are attracted to the nucleus of an atom. In a nucleophilic substitution reaction, the nucleophile reacts with a haloalkane. The haloalkane has a halogen atom bonded to the central carbon atom and the halogen atom is displaced by the nucleophile. This halogen atom leaves as a halide ion and therefore, termed a leaving group. The alkoxide ion, the hydroxide ion, the halide ion, and the cyanide ion are common examples of nucleophiles. Nucleophilic substitution reactions are of two types – SN1 and SN2.
Leaving Group
In a nucleophilic substitution reaction, a molecule should have a good leaving group as well as a polar bond to act as a nucleus or a substrate. An atom that has the capability of independent existence and can exist as an ion or molecule, which is weakly basic and relatively stable, can act as a good leaving group. A good leaving group should have a high electronegativity so that it can accommodate a negative charge. The negative charge can also be accommodated through delocalization. An example of a good leaving group is halogens since they form relatively stable ions and have high electronegativity.
SN1 reaction
This substitution reaction takes place in a two-step process.
In the first step, a positively charged carbon (carbocation) is formed when the carbon-halogen bond breaks.
In the second step, a new bond is formed when the nucleophile attacks the carbocation, that is, the carbon ion with a positive charge.
This reaction is a unimolecular reaction.
It follows first-order kinetics.
The rate of the reaction can be written as k[R-LG].
The concentration of the alkyl halide, that is, the substrate determines the rate of the reaction.
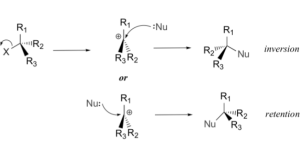
Both inversion and retention products are formed, that is, the reaction has a racemization stereochemistry.
The reactivity of the reaction can be increased by using a polar protic solvent.
The reaction mostly takes place in secondary and tertiary haloalkane.
The nature of the leaving group affects the rate of the reaction.
The rate of the reaction is not affected by the nature of the nucleophile.
A leaving group that can easily be removed and is a weak base is considered as a good leaving group.
SN2 reaction
This substitution reaction takes place in a one-step process.
The formation of a carbanion occurs simultaneously when the nucleophile bonds with the central carbon atom and the breaking of the carbon-halogen bond.
This reaction is a bimolecular reaction.
It follows second order kinetics.
The rate of the reaction can be written as k[Nu][R-LG].
The concentration of the alkyl halide, that is, the substrate as well as the nucleophile determines the rate of the reaction.
The reaction shows an inversion stereochemistry.
The reactivity of the reaction can be increased by using a polar aprotic solvent.
The reaction mostly takes place in primary and secondary haloalkanes.
The nature of the leaving group affects the rate of the reaction.
The reaction is also affected by the nature of the nucleophile.
A leaving group which is a weak base can be considered as a good leaving group.
Nucleophilic substitution reaction example
Example of an SN1 reaction:
In the first step, the leaving group moves out. Here the leaving group is denoted by ‘X’. When the C-X bond breaks, the positive charge on the carbon atom is formed (carbocation). The first step can be illustrated as follows:
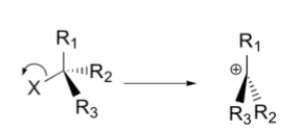
In the second step, a nucleophile attacks the central carbon atom and forms a bond. The configuration of the product may be an inversion or retention based on the face where the nucleophile is oriented. The second step can be illustrated as follows:
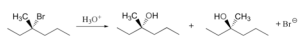
The following is an example of an SN1 reaction on (S)-3-Bromo-3-methyl hexane. This is a tertiary alkyl halide.
Example of an SN2 reaction:
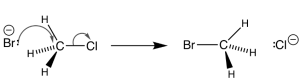
In an SN2 reaction, there is a simultaneous bond formation between a nucleophile and central carbon atom and the breaking of the bond between carbon and the leaving group. The SN2 reaction can be illustrated as follows:
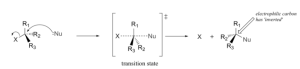
The following is an example of SN2 substitution on methyl chloride. The leaving group here is Chlorine and the nucleophile is Bromine
Conclusion
When an electron pair donor (nucleophile) reacts with an electron pair acceptor, the reaction is termed a nucleophilic substitution reaction. The nucleophile displaces the halogen atom bonded to the central carbon atom of the haloalkane. The halogen which leaves is termed the leaving group. This reaction can be categorised into two types – SN1 and SN2.
The SN1 reaction is a two-step reaction, and as the name suggests, is a unimolecular reaction (hence 1). In the first step, the bond between carbon and the halogen breaks and in the second step, the nucleophile bonds with the central carbon atom.
The SN2 reaction is a one-step reaction, and as the name suggests, is a bimolecular reaction (hence 2). In this reaction, there is a simultaneous bond formation between carbon and the nucleophile and breaking the bond between carbon and halogen.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





