Rubber is an elastomeric – elastic polymer – material that comes in many forms. It may be sourced from tropical plants in the form of natural rubber, as well as from petroleum and natural gas in the form of synthetic rubber.
Rubber is flexible and durable, and therefore, has many uses. Rubber is the primary material used in manufacturing of tyres used in vehicles such as cars and bicycles.
Along with tires in the automobile industry, rubber is also used in the manufacture of mechanical items such as mountings, gaskets, belts, hoses, and consumer goods such as shoes, clothing, furniture, and toys.
Elastomers, short for elastic polymers, are the main chemical components of rubber. Elastomers are long chainlike molecules that can stretch to vast distances and return to their original shape.
Rubber
We are surrounded by rubber in our day-to-day lives. We encounter rubber in gloves, bands, boots, and even erasers. Rubber is such a popular raw material because it can return to its original shape after being stretched or twisted. This is why it is categorised as an elastomer. Rubber is an elastic material that can be obtained either naturally or artificially.
Rubber comes in two forms: natural rubber and synthetic rubber.
Natural Rubber
Plants create natural rubber, which is categorised as a polymer.
A polymer is a chemical substance made up of many smaller molecules of the same type. While some polymers are found in nature, others are manufactured in laboratories and factories.
Natural rubber is a flexible material manufactured from the latex sap of trees, especially those from the Hevea and Ficus species.
Natural rubber is an elastomer or an elastic hydrocarbon polymer. Natural rubber is one of the varieties of rubber, along with Vulcanised rubber, which is used to make various rubber products. Indian rubber, gum elastic, and caoutchouc are all names for natural rubber.
The chemical formula for natural rubber is derived from its monomer, 2-methyl-1,3-butadiene (isoprene). Its formula is CH2=C(CH3)-CH=CH2.
The polymerisation reaction for natural rubber is
nCH2=C(CH3)-CH=CH2 → -[CH2-C(CH3)=CH-CH2]n–.
Synthetic Rubber
Synthetic rubbers were being made as early as 1961 by catalysing vanadium. The resultant polymer could be used in cables and wires. The toughness and elasticity of rubber were particularly useful in electrical appliances.
Synthetic rubbers have also been widely used in O-rings and braking applications since the 1970s.
Synthetic rubber is produced by adding polymers of polyene monomers/ After that, they are laminated using additional polymers.
Synthetic rubber can be used instead of natural rubber as a cheaper and more durable alternative. Examples of synthetic rubbers include buna rubbers, butyl rubbers and neoprenes.
Synthetic rubbers are sometimes preferred compared to natural rubbers because they are easier to manufacture. They are used in tyres, along with in other objects such as elevator belts, hoses, tubes, and seismic bearings. They are also used by the consumer goods industries to produce footwear, sports equipment, erasers, etc. Another kind of synthetic rubber named polyisoprene has identical properties to natural rubber.
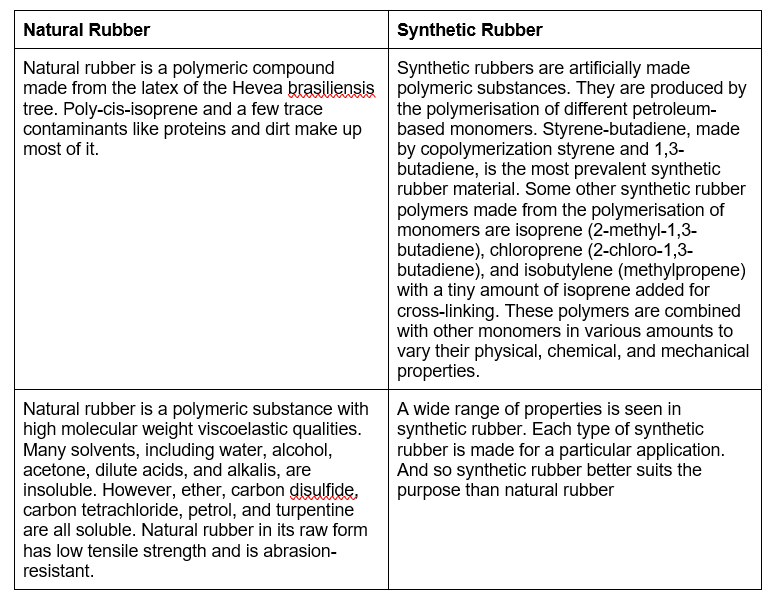
Difference between Natural Rubber and Synthetic Rubber
Vulcanisation of Rubber
Vulcanisation is a chemical process that involves heating rubber with sulphur, accelerator, and activator at 140–160 degrees Celsius. Cross-linking between long rubber molecules improves resilience, elasticity, viscosity, tensile strength, weather resistance, and hardness.
There are two methods of Vulcanizing Rubber
Vulcanisation under Pressure
This is the most common process of Vulcanizing rubber. It involves heating rubber with sulphur and applying pressure at 150 degrees Celsius. Other chemicals and agents, such as fillers to boost strength and resilience to wear and tear, or carbon black to act as a reinforcer, is also used in the process.
Unrestricted Vulcanization
Unrestricted Vulcanization, also known as free Vulcanization, refers to the process of Vulcanizing rubber by running hot steam through it. This method of Vulcanization takes around eight hours to complete. However, chemical activators can be added to speed up the process.
Conclusion
Natural rubber and synthetic rubber both have excellent tear resistance, low-temperature flexibility, and tensile strength. Natural rubber provides several advantages over synthetic rubber, including superior tensile strength, tear resistance, and minimal odour as compared to synthetic rubber.
However, particular uses can be discovered in various synthetic rubbers as well. Chemical, fluid, ozone, electrical, and other types of resistances can all be found in synthetic rubbers. Synthetic rubbers can also have high heat resistance, low-temperature resistance, and better heat ageing properties.
Another factor to consider when deciding between natural and synthetic rubber is that natural rubber includes natural proteins that might induce allergic reactions when exposed to human skin for a lengthy period. Despite the variations between natural and synthetic rubber, manufacturers want low costs and good performance in their applications.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out