Introduction
We as a whole need to realize that in a specific substance the number of particles is available. Particles and atoms are extremely little, both in size and mass. The molar mass of a substance refers to the total mass of one mole of a substance. Associate the nuclear masses (nuclear loads) of all particles inside the atom to ascertain the molar mass. Observe the nuclear mass for every component utilizing the mass displayed in the Periodic Table or Atomic Weight Table.
Duplicate the addendum (number of atoms) times that component’s nuclear mass and add the majority of the relative multitude of components in the particle to acquire the atomic mass. Molar mass is normally communicated in either gram ( g) or kilograms (kg).
Molecular weight, likewise called sub-atomic mass, is the mass of a particle of a substance, in view of 12 as the nuclear load of carbon-12. It is determined practically speaking by adding the nuclear loads of the particles making up the substance’s atomic recipe. The sub-atomic load of a hydrogen particle (synthetic equation H2) is 2 (subsequent to adjusting); for some intricate natural particles (e.g., proteins, polymers) it very well might be in large numbers.
Molar mass is the mass of one mole of a given compound or substance. We realize that the number of moles of a compound (n) is the proportion of the given mass of the compound to the molar mass of the compound and can be communicated as follows:
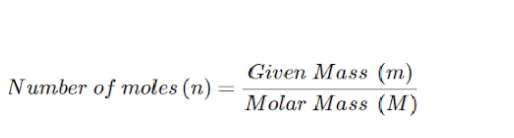
We can compose the above equation in an accompanying manner to get the molar mass (m) of the compound.
⇒Molar Mass (M)=Mass (m)×Number of moles(n)
Implies molar mass (M) is the result of mass (m) of the compound and the number of moles (n) of the given compound.
The unit to quantify the mass of the compound is the gram. The unit to quantify the molar mass of the compound is grams per mole or g/mol.
The molar mass is characterized as the mass of 1mol, which is in the SI units estimated in g/mol.
Example: The molar mass of water H2O: MM=18.016g/mol.
mole, also spelled mol, in science, is a standard coherent unit for assessing gigantic measures of small substances like particles, molecules, or other demonstrated particles.
The mole assigns a very enormous number of units, 6.02214076 × 1023. The General Conference on Weights and Measures characterized the mole as this number for the International System of Units (SI) viable from May 20, 2019. The mole was recently characterized as the quantity not really set in stone tentatively to be found in 12 grams of carbon-12. The quantity of units in a mole additionally bears the name Avogadro’s number, or Avogadro’s steady, out of appreciation for the Italian physicist Amedeo Avogadro (1776–1856). Avogadro suggested that equivalent volumes of gasses under similar conditions contain a similar number of particles, a theory that demonstrated helpful in deciding nuclear and sub-atomic loads and which prompted the idea of the mole.
The quantity of atoms or different particles in a mole is something very similar for all substances. The mole is identified with the mass of a component in an accompanying manner: one mole of carbon-12 particles has 6.02214076 × 1023 iotas and a mass of 12 grams. In the examination, one mole of oxygen comprises, by definition, a similar number of iotas as carbon-12, yet it has a mass of 15.999 grams. Oxygen, consequently, has a more prominent mass than carbon. This thinking additionally can be applied to atomic or equation loads.
The idea of the mole assists with putting quantitative data concerning what occurs in a synthetic condition on a perceptible level. For instance, in the synthetic response 2H2O → O2 + 2H2, two moles of water are decayed into two moles of atomic hydrogen and one mole of sub-atomic oxygen. The mole can be utilized to decide the least complex recipe of a compound and to work out the amounts associated with synthetic responses. When managing responses that occur in arrangements, the connected idea of molarity is helpful. Molarity (M) is characterized as the number of moles of a solute in a liter of arrangement.
Molecular weight is a proportion of the amount of the nuclear weight upsides of the particles in a particle. Atomic weight is utilized in science to decide stoichiometry in synthetic responses and conditions. Atomic weight is regularly abridged by M.W. or on the other hand MW. Sub-atomic weight is either unitless or communicated as far as nuclear mass units (amu) or Daltons (Da).
Both atomic weight are characterized comparative with the mass of the isotope carbon-12, which is appointed a worth of 12 amu.
The reason the atomic weight of carbon is not precisely 12 that it is a mixture of isotopes of carbon.
Sample Molecular Weight Calculation
The calculation for molecular weight depends on the sub-atomic equation of a compound (i.e., not the least complex recipe, which just incorporates the proportion of sorts of particles and not the number). The quantity of each kind of iota is increased by its nuclear weight and afterward added to loads of different particles.
For instance, the atomic equation of hexane is C6H14. The addendums demonstrate the quantity of each kind of iota, so there are 6 carbon particles and 14 hydrogen iotas in every hexane atom. The nuclear load of carbon and hydrogen might be found on an intermittent table.
- Atomic weight of carbon: 12.01
- Atomic weight of hydrogen: 1.01
molecular weight = (number of carbon atoms)(C atomic weight) + (number of H atoms)(H atomic weight) so we calculate as follows:
- the molecular weight = (6 x 12.01) + (14 x 1.01)
- hexane’s molecular weight is= 72.06 + 14.14
- hexane’s molecular weight is = 86.20 amu
Conclusion
How Molecular Weight Is Determined
Exact information on the sub-atomic load of a compound relies upon the size of the particle being referred to. Mass spectrometry is ordinarily used to track down the sub-atomic mass of little to medium-sized particles The heaviness of bigger atoms and macromolecules (e.g., DNA, proteins) is tracked down utilizing light dispersing and consistency In particular, the Zimm strategy for light dissipating and the hydrodynamic techniques dynamic light dispersing (DLS), size-avoidance chromatography (SEC), dissemination requested atomic attractive reverberation spectroscopy (DOSY), and viscometry might be utilized.
molecular Weight and Isotopes
Note, assuming you are working with explicit isotopes of a particle, you should utilize the nuclear load of that isotope rather than the weighted normal given from the intermittent table. For instance, if rather than hydrogen, you are managing the isotope deuterium, you utilize 2.00 rather than 1.01 for the nuclear mass of the component. Conventionally, the contrast between the nuclear load of a component and the nuclear load of one explicit isotope is generally minor, however, it very well may be significant in specific computations!
Molecular Weight Versus Molecular Mass
Molecular weight is frequently utilized reciprocally with sub-atomic mass in science, albeit in fact there is a distinction between the two. Sub-atomic mass is a proportion of mass and sub-atomic weight is a proportion of power following up on the sub-atomic mass. A more right term for both sub-atomic weight and sub-atomic mass, as they are utilized in science, would be “relative sub-atomic mass”.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





