Elements constantly try to get eight electrons in the outermost or the valence shell. In order to get all eight electrons in their respective valence shells, atoms form bonds with their most compatible atoms either by donating or sharing electrons. A metallic bond can be described as a bond where there are several metallic atoms in the metallic substance. Every time an atom loses an electron it becomes a positive ion which later gives rise to the cohesive binding force which holds all crystals together.
In its free state, the metal does not exist as a single atom as it either forms metallic bonds or ionic bonds with similar atoms. Not every metal leads to the formation of metallic bonds when they exist in the free state. For example, mercury forms the metal-metal covalent bond when it exists in the free state. Today, we are going to discuss the concept of metallic bonding importance in detail. So, without any further delay, let’s get started!
What is Metallic Bonding?
The metallic bond is a chemistry term used for describing the overall sharing of the sea of the Valance electrons between multiple positively charged ions of metal. Metallic bonding can be defined as a common type of chemical bonding which is responsible for several metal properties, including their malleability, their conductivities for electricity and heat, and their shiny lustre.
Example – Metallic bonding in the sodium
The sodium’s electron configuration is 1s22s22p63s1. It mainly has a single electron in its valence shell. Metallic sodium features several Na+ ions in the solid-state, which are surrounded by the sea of 3s electrons.
Here is an illustration showing the metallic bonding importance in the sodium –
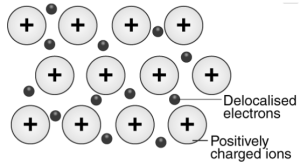
What are Metals?
Metals can be defined as substances or minerals formed naturally on the earth’s surface. Mostly, metals are shiny and lustrous; however, they are made of components that were never alive on the earth; therefore, metals are inorganic. Generally, metals are found in rocks washes by groundwater, surface water, or atmospheric dust.
Metals are extremely durable and strong; as a result, it is nearly impossible to change their shapes using hands. Several things are made up of metals. These include cooking utensils, satellites, automobiles, etc. Certain metals such as sodium and potassium are not as hard as other metals; as a result, they can be cut using a knife. In the world of metals, mercury is an exception as it is in the liquid state at room temperature.
The extraction and isolation of metals take place over several major steps. These include –
- Ore concentration
- Metal isolation from concentrated ore
- Metal purification
Here are the steps involved in the metal extraction from ores-
- Crushing and Grinding
Metals are generally found in big chunks inside the earth’s crust. In the first metal extraction step, the ores are crushed and grounded in crushers and ball mills. It increases the chunk’s surface area, which results in better chemical action. This method is known as pulverisation.
- Concentrating Ore
The second step involves concentrating the ore, which is related to the removal of impurities. This process is known as ore dressing that involves several methods such as Hydrolytic Method, Magnetic Separation, Froth Floatation, and lastly, Chemical Separation.
- Roasting and Calcination
Once the ore is finely concentrated, it is then heated in the absence or the presence of the air, depending on the chemical properties of the extracted metals. For example, sulphide ores are mostly heated in the presence of oxygen, whereas carbonated metal ores are heated in the presence of a vacuum.
Properties Attributed by Metallic Bonding
Metallic bonding features an array of properties that makes them desirable. Here are some of the properties attributed to metallic bonding –
- Electrical Conductivity
Electricity conductivity can be described as the substance’s ability to move charge through it. Since the electron movement is not restricted, the current can easily pass through the metal. As the potential difference gets introduced to metals, delocalised electrons move towards positive charges, which is why metals are great conductors of electric current.
- Thermal Conductivity
The material’s thermal conductivity can be described as its ability to transfer or conduct heat. Every time an end of the metallic substance is heated, it leads to a rise in the electron’s kinetic energy, which later gets transferred to other electrons through collisions.
- Malleability and Ductility
When the ionic crystal including sodium chloride crystal gets beaten through the hammer, it breaks down into smaller pieces. The force-induced through the hammer leads to fracture of the crystal structure leading to shattering of the crystal.
- High Melting and Boiling Points
Metals generally have high melting and boiling points as attractive force laying between the metal atoms is pretty strong. To overcome this force, a great amount of energy is needed as a result, metals possess high boiling and melting points. The exceptions are cadmium, zinc, and mercury.
Conclusion
With this, we come to an end of the topic What is Metallic Bonding. Metals as substances or minerals are formed naturally on the earth’s surface. Mostly, metals are shiny and lustrous; however, they are made of components that were never alive on the earth; therefore, metals are inorganic. They are found in rocks washes by groundwater, surface water, or atmospheric dust. Metallic bonding is a common type of the chemical bonding which is responsible for several metal properties including their malleability, their conductivities for electricity and heat, and their shiny lustre. We ended the topic with four major properties of metallic bonding and metallic bonding importance.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



