Introduction
Alkanes undergo very few reactions. One is being halogenated by single hydrogen substituted for a single halogen forming haloalkane. The halogenation of alkanes takes place in the presence of photochemical conditions.
Let us assess an example of halogenation of alkane-the chlorination of methane, to understand the mechanism of halogenation. Nothing happens when methane(CH4) and chlorine (Cl2) are fused in the absence of light at room temperature. But, if the conditions are changed, a reaction is taking place at high temperatures or there is ultraviolet irradiation, a product is formed, chloromethane(CH3Cl).
Halogen-containing compounds are common, making the transformation important in the production of polymers and drugs. Halogens are also commonly introduced using salts of halides and halogen acids. Many specialized indicators introduce halogens into diverse substrates like thionyl chloride and chlorination of gold forms gold(III) chloride. Halogenation is influenced by halogens.
Body
Radical Chain Mechanism
- The proceeding of reactions takes place through a radical chain mechanism.
- The halogenation of alkanes mechanism is divided into 3 steps:
- Initiation
- Propagation
- Termination
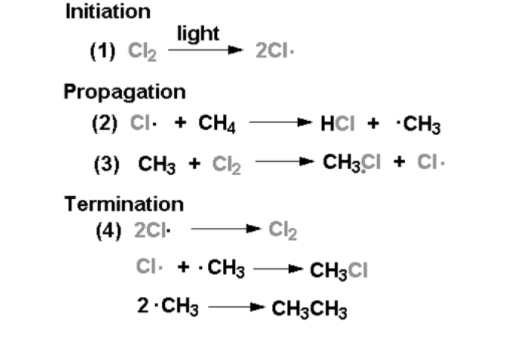
Initiation:
The bond between the chlorine molecule (Cl2) breaks due to initiation. This step is not energetically favorable, for this step energy must be put in. After this step, without the input of more energy, the reaction can occur continuously (as long as reactants provide). The external energy input occurs through heat and light.
Propagation:
The next two steps are known as propagation steps-
Step 1: A chlorine radical combines with hydrogen on the methane. This combination gives out methyl radical and hydrochloric acid (HCl is an inorganic product of this reaction). This step is endothermic. It is not energetically favorable and takes in heat (requires 2kcal/mol).
Step 2: More of the chlorine starting material (Cl2) is used. One chlorine atom becomes a radical while the other combines with the methyl radical. This step is exothermic. It occurs swiftly, releasing 27kcal/mol. This step uses the product of step-1, the methyl radical.
Termination:
All the radicals combine together to form more product (CH3Cl), more reactant (Cl2), and more such combinations of the two methyl radicals forming a side product of ethane (CH3CH3).
- The reaction doesn’t stop after one chlorination.
- It is difficult to get monosubstituted chloromethane. Rather di-, tri- and even tetra- chloromethane are formed.
- To avoid this difficulty, one must use a higher concentration of methane as compared to chloride.
- Initially, this free-radical chain reaction contains few free radicals and many molecules of reactants.
- As the reaction progresses, the number of free radicals increases with a decrease in the number of reactants.
- Nearing the end, we’ve many more free radicals than reactant molecules.
- Overall, the termination step becomes the predominant reaction.
- Altogether, halogenation mechanism reactions occur swiftly, and the formation of the products takes only microseconds.
How is halogenation controlled?
Halogenation of alkanes never stops at mono-substitution.
The chlorination of methane gives rise to dichloromethane, chloroform, and carbon tetrachloride but depending on the reaction.
By Controlling the concentration of halogen, mono , di , tri or tetra halogenated products are possible.
There exists more than one possible product of hydrocarbons depending on the replacement of hydrogen. For example, Butane (CH3-CH2-CH2-CH2Cl) can be chlorinated at position 1 giving 1-chloro-butane (CH3-CH2-CH2-CH2Cl) or at position 2 giving 2- chloro-butane (CH3-CH2-CHCl-CH3). The product depends on reactive rate- position 2 of butane reacts swiftly giving the major product 2-chloro-butane.
Bromination>Chlorination>Fluorination (> means ‘less selective’).
Some other important points
- Fluorination is least selective yet is highly exothermic and special attention is needed to avoid explosion or a runaway reaction. This relationship can be elucidated with the help of the Hammond postulate and is seen as a demonstration of the relativity-selectivity principle.
- The transition state of hydrogen abstraction has a radical character and is reached late while a bromine radical is not very reactive.
- The transition state of reactive chlorine radical resembles the reactant with radical character.
- The alkyl radical can benefit from any resonance stabilization and is fully formed in a transition state thereby maximizing selectivity.
- The selectivity of bromination is understood through Bond Dissociation Energies(BDEs). The BDE is the energy of a bond required to break it with the help of homolytic cleavage. It further determines if the reaction is exothermic or endothermic.
- No reaction between alkanes and iodine takes place as iodine does not react with alkane.
- No reaction between alkane and chlorine or bromine in the dark but if the light/heat is present then the required alkyl halides can be produced.
For the other halogens, free-radical halogenation takes place in the following order:
(> reacts faster than)
Carbons with one or more aryl substituents (benzylic positions) > Carbons with three alkyl substituents (tertiary positions) > Carbons with two alkyl substituents (secondary positions)>Carbons with one or zero substituents (primary positions).
Halogenation inhibitor- OXYGEN.
Halogenation of aromatic compounds typically works well for chlorine and bromine.
Aromatic compounds are subject to electrophilic halogenation.
RC6H5+X2 → HX+RC6H4X
Balz-Schiemann reaction-To prepares fluorinated aromatic compounds.
Conclusion
Alkanes undergo very few reactions. One is being halogenated by single hydrogen substituted for a single halogen forming haloalkane. The halogenation of alkanes takes place in the presence of photochemical conditions. The halogenation of alkanes mechanism is divided into 3 steps: Initiation, Propagation and Termination. The selectivity of bromination is understood through Bond Dissociation Energies(BDEs). The BDE is the energy of a bond required to break it with the help of homolytic cleavage. It further determines if the reaction is exothermic or endothermic.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





