In organic chemistry, several kinds of elements like – alkenes, alkynes, etc. Each element exhibits different behavior and has different properties. Alkenes undergo different reactions. An addition reaction forms a strong reaction between two or more molecules that interact with each other. The resultant molecule is called the adduct. In organic chemistry two types of reactions take place mainly. These are-
- Electrophilic Addition Reaction
- Nucleophilic Addition Reaction
Electrophilic Addition Reaction: It is an addition reaction in which a substrate is initially attacked by an electrophile with the formation of a chemical compound containing two new sigma (σ) bonds and has a broken double or triple pi(π) bond.
Alkenes constitute a group of unsaturated hydrocarbons containing at least one double bond. As a result of the double bond, alkenes undergo an addition reaction. When an electrophile attacks with the double bond of carbon atoms with the help of pi electrons present in the alkenes.
Electrophilic Addition Reaction Mechanism
Step 1- In first step of Electrophilic addition reaction mechanism, the 2 pi electrons from double bond attack the H in the HX (X= Cl, Br, I) electrophile. The 2 pi electrons form a C-H sigma bond between the H from HX and a carbon from the double bond.
Simultaneously, the electrons from the H-X bond move onto the halogen to form a halide anion. This step is known as the deprotonation step.
Step 2- The carbocation formed acts as an electrophile and accepts a pair of electrons from the nucleophilic halide ion. The electron pair becomes a X-C sigma bond by neutralizing the alkyl halide product of electrophile addition.
All halides (HBr, HCl, HI, HF) participate in this reaction and add on in the same manner but different halides have different rates of reaction, as H-X bond gets weaker as X increases due to poor overlapping of orbitals.
In general, hydrogen halides are represented as –
HI>HBr>HCl
Electrophilic Addition Reaction of Alkenes
Alkenes exhibit a wide range of electrophilic addition reactions. Addition of hydrogen halides such as HBr is an example of electrophilic addition reactions of alkenes.
For symmetrical alkenes such as ethene, it is easy to predict the end product as compared with the unsymmetrical alkenes such as propene.
For Example-
CH2=CH2+ H-Br → CH3-CH2-Br
Electrophilic Addition Reaction rate varies by following factors-
- Change in Halogen:
Bond strength falls as we go from HF to HI whereas the reaction rates increase from HF to HI. The H-F bond is strong and cannot be easily broken.
- Change in Alkene:
As the number of alkyl group increases attached to carbon atom at either end of the double bond, the rate reaction increases.
Examples for Electrophilic Addition Reaction of alkenes mechanism are-
- Hydrogenation
- Halogenation
- Cyclopropanation
- Hydroxylation
- Hydration
- Oxidation
Oxidation reaction of alkenes
Formation of ketones and alcohols using Electrophilic Addition Reactions:
Formation of ketones and alcohols occurs using Electrophilic Addition Reaction in the oxidizing state by using potassium permanganate. If the potassium permanganate is in an acidic state, then the ketone is produced as a result of oxidation whereas if the potassium permanganate is in aqueous state, then the alkenes will get oxidized and produce the glycols.
Nitration Mechanism:
Nitration is the substitution of the nitro group into the aromatic ring. Nitration is the aromatic substitution reaction. It is a mechanism of electrophilic substitution reaction. Substitution involves an electrophile addition.
Its mechanism is as follows:
Step 1- An electrophile is generated in the first step. The first step is slow and reversible. Due to breakage of bond, the process gets slowed down as a result of which a barrier of activation energy is formed.
![]()
Step 2 – Sigma(σ) complex is formed in the next step. It is a fast step in the reaction process. A stable aromatic compound converting into the unstable product is formed. It is a reversible step as products can recombine to form the products. HSO-4 basic in form deprotonates the complex form.
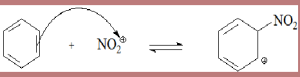
Step 3 – Re-aromatization
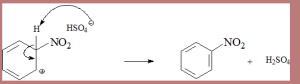
Mechanism of Electrophilic Substitution Reaction:
An Electrophilic Substitution Reaction is a reaction in which the functional group is replaced by an electrophile attached to the chemical compound. The functional group is mainly H-atom. Electrophilic substitution reaction is mainly a 3-step mechanism process.
The following steps are –
- Generation of an electrophile- Aluminium chloride acts as a lewis acid in the generation of an electrophile from the process of chlorination, alkylation, and acylation. This results in the generation of following electrophiles – Cl+, R+, and RC+O respectively.
- Carbocation formation (Intermediate step)- A sigma complex or arenium ion is formed when an electrophile attacks the ring. The arenium ion becomes stable in a resonance structure. The aromatic character is lost with the delocalization of sp3 hybridized carbon.
- Removal of proton form intermediate- To restore the lost aromatic character, proton is released from the sigma complex (sp3 hybridized) when [AlCl4]-. Hence, electrophile replaces the hydrogen atom in the benzene ring.
Types of Electrophilic Substitution Reactions –
- Electrophilic Aromatic Substitution Reactions: In an electrophilic aromatic substitution reaction, an atom is replaced by the electrophile attached to the aromatic ring. The aromaticity of the compound is conserved in the electrophilic aromatic substitutions. Thus, this type of rection is used in obtaining aryl halides from aromatic rings and iodine, bromine or chlorine.
For e.g.- Aromatic nitrations, sulphonation, Friedel- Crafts reactions.
- Electrophilic Aliphatic Substitution Reactions: In an electrophilic aliphatic substitution reaction, an electrophile replaces the functional group. The functional group is generally a hydrogen atom. The electrophilic substitution results in an inversion of configuration when attacked by the electrophile occurring at an angle of 180o with the leaving group.
This reaction has been classified into following types-
1.Halogenation of ketones
- Nitrosation
- Keto-Enol tautomerism
- Carbene into a C-H bond
- Diazonium coupling
Conclusion
These are the several organic chemical reactions that can be formed using alkenes in electrophilic addition reactions. Each element in a periodic table reacts in a different manner at various states as in the case of oxidation reaction. So, each reaction has its own importance in everyday life and for commercial purposes so it is important to understand its mechanism as well.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





