Ketones are found all over the world and are frequently coupled with other functional groups. The group can be found in almost all biological substances. Carbohydrates, lipids, proteins, nucleic acids, hormones, and vitamins are examples of organic substances important to living systems. Two carbon groups are connected to the carbonyl carbon atom in a ketone. Ketones are represented by the following general formulae, where R represents an alkyl group, and Ar represents an aryl group.
The position of the carbonyl group inside the molecule distinguishes aldehydes from ketones. An aldehyde is an organic molecule with a carbonyl group linked to a carbon atom at the carbon chain’s terminus. A ketone is an organic molecule that has a carbonyl group linked to one of the carbon atoms in the carbon chain.
Methanol or formyl is a term used to describe aldehydes and ketones. This group’s carbon atom contains two leftover bonds that might be filled by aryl, alkyl, or substituents. The compound is a Ketone if none of these substituents is hydrogen. An aldehyde is a chemical with at least one hydrogen atom.
Naming of Ketones
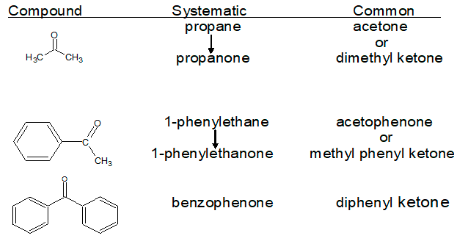
For aldehydes and ketones, both common and International Union of Pure and Applied Chemistry (IUPAC) names are commonly used, with common names predominating for the lower homologs. The names of the acids into which aldehydes can be transformed by oxidation are used as common names for aldehydes. Ketones have a distinguishing suffix of -one in the IUPAC naming system. A ketone carbonyl function can be found at any point along a chain or ring, and its location is generally indicated by a number. The numbering of a chain usually begins at the end closest to the carbonyl group. Because there is only one potential site for a ketone carbonyl function, very simple ketones like propanone and phenylethanoid do not require a locator number.
Ketones are given common names by first identifying both alkyl groups linked to the carbonyl and then adding the suffix -ketone. The connected alkyl groups are listed alphabetically by name.
Ketones get their name from the alkane chains that they come from. The ending has been replaced with -one. The alphabetical list of substituent groups + ketone is the popular term for ketones. The generic names of certain common ketones are well-known. For example, propanone is frequently referred to as acetone.
Properties of Ketones
Aldehydes and Ketones can form a weak hydrogen bond with water by the carbonyl oxygen atom, which is present in it. Both series’ lowest members ( having 3 or lesser numbers of carbon) are water-soluble in all amounts. Water solubility reduces as the carbon chain length rises. Like ethers, Aldehydes and ketones cannot form hydrogen bonds with one another. As a consequence of this, their boiling points are lower than those of alcohol in general. Aldehydes and ketones, unlike alkanes, are polar compounds with a more electronegative oxygen atom. The dipole-dipole interactions in Aldehydes and Ketone are stronger than those of the dispersion forces present in the alkanes. Aldehydes and ketones have boiling points that are halfway between alkanes and alcohols. Ethane, for example, has a boiling point of 89°C, ethanal is 20°C, and ethanol has a boiling point of 78°C.
The carbon-to-oxygen double bond is very polar, even more so than the single bond. The bonding electron pairs are far more attracted to the electronegative oxygen atom than they are to the carbon atom. A partial positive charge exists on the carbon atom, while a partial negative charge exists on the oxygen atom.
Common Names of Some Ketones
Some common names are still in use, and they must be learned. It might be beneficial to recognise trends.
Uses of Ketones
- Acetone, the most common ketone, is a great solvent for a variety of polymers and synthetic fibres.
- Acetone is commonly used in the home as a nail polish remover and paint thinner.
- It’s utilised in chemical peeling and acne treatments in medicine.
- A typical solvent is methyl ethyl ketone (MEK), also known as butanone. Textiles, varnishes, plastics, paint remover, paraffin wax, and other products include it.
- Because of its dissolving qualities, MEK is frequently employed as a plastic welding agent.
- Another major ketone is cyclohexanone, which is largely utilised in the manufacture of nylon.
Some of the Common Ketones
Acetone, methyl ethyl ketone, and cyclohexanone are the most important ketones on a scale of importance. These are also very frequently used in biochemistry, but not as much as they are in organic chemistry. The simplest ketone is dimethyl ketone (CH3COCH3), often known as acetone. Acetone is a clear liquid with no colour. It’s used as a solvent for lacquer (including fingernail polish), cellulose acetate, cellulose nitrate, acetylene, plastics manufacturing, as paints and as a remover of the varnish and also as a solvent in pharmaceutical and chemical manufacturing.
Acetone is prepared from propylene by direct or indirect method. The cumene process produces around 83 per cent of acetone; as a result, acetone manufacturing is linked to the synthesis of phenol. Cumene is produced by alkylating benzene with propylene, which is then oxidised by air to create phenol and acetone. Other methods include directly oxidising propylene (Wacker-Hoechst process) or hydrating propylene to produce 2-propanol, which is then oxidised to acetone.
Reactions of Ketones
Ketones are extremely reactive, but not as much as aldehydes, with which they have a tight relationship. The nature of the carbonyl group is responsible for most of its chemical activity. Ketones are easily reached within a wide range of chemical reactions. The carbonyl group is extremely polar, which means it has an unequal distribution of electrons. This is one of the main reasons. This provides the carbon atom with a partial positive charge, making it vulnerable to assault by nucleophiles.
Conclusion
A ketone is an organic molecule that has a carbonyl group linked to one of the carbon atoms in the carbon chain. The names of the corresponding carboxylic acids are used to give aldehydes their common names: formaldehyde, acetaldehyde, and so on. Ketones, like others, have common names that include the names of the groups linked to the carbonyl group, followed by the term ketone.
Aldehydes and ketones have stem names that are derived from the parent alkanes, with a -al ending for aldehydes and a -one ending for ketones.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





