Intramolecular hydrogen bonding is also responsible for making the secondary or tertiary proteins structures and structures for nucleic acids to some extent. It also plays a critical role in deriving polymers’ synthetic or natural structure.
Meaning of Intramolecular Bonding
Intramolecular bonding is generally associated with the formation of molecules. Also called primary bonds, these are attractive forces between atoms or ions within molecules (or formula units). These forces affect the macroscopic properties and chemical properties of substances.
Microscopic properties are properties of individual particles—for example, molecular mass, molecular structure, bond length, bond angles, and bond energy. Primary bonds are much stronger in nature rather than secondary bonds.
It is easy to define primary bonds by defining strong attractions among atoms within a single molecule that further includes the exchange of valence electrons. These forces include ionic bond, covalent bond, coordinate covalent bond, and metallic bond.
Intramolecular Hydrogen Bonding
These kinds of bonds occur within the atom of one single bond. When mainly two groups of molecules make hydrogen bonds, this happens. Both a hydrogen donor and a hydrogen acceptor must be present within the same molecule, and they must be close to each other for this to happen.
Because of the nature of molecular geometry, intramolecular hydrogen bonding occurs between the two hydroxyl sets in ethylene glycol (C2H4(OH)2).
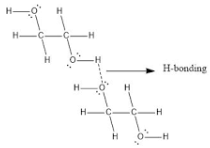
Simply said, it happens when a hydrogen atom is sandwiched between two strongly electronegative (F, O, N) atoms in the same molecule. In o-nitrophenol, for example, the hydrogen atom is sandwiched between the two oxygen atoms.
To better understand the forms of hydrogen bonding and branches of intramolecular hydrogen bonding, let’s look at the many types of molecular forces in polymers.
Molecular Forces in Polymers
Atoms are held collectively in molecules by different bonds based on the valence electrons. Molecules are attracted to each other by weaker bonds, which generally result from the electron configuration of the individual molecules. Thus, we have broadly two types of bonding:
- Primary Bonds/Bonding due to Intramolecular forces
- Secondary Bonds/Bonding due to Intermolecular forces
Conclusion
When a hydrogen atom connected to a strongly electronegative atom is near another electronegative atom with a lone pair of electrons, it creates a specific sort of dipole-dipole attraction called a hydrogen bond. Intermolecular forces (IMFs) are the forces that exist between molecules. Ordinary dipole-dipole interactions and dispersion forces are some examples. Normal dipole-dipole and dispersion forces are greater than hydrogen bonds, while genuine covalent and ionic bonds are weaker.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



