Hydrogen bonds can be classified into two categories: one is intermolecular hydrogen bonding (processing between separate molecules) and the other is intramolecular hydrogen bonding (processing within parts of the same molecule).
Many of the abnormal physical and chemical properties of the compounds of N, O, and F are derived based on the hydrogen bond. Specifically, the high boiling point of water at (100 °C) compared to the other hydrides that have much weaker hydrogen bonds is mainly because of the intermolecular hydrogen bonding.
Intermolecular Hydrogen Bonding
Intermolecular hydrogen bonding can be seen between separate molecules in a substance . They can occur between any number of the same or unlike molecules as far as the hydrogen donors and acceptors are available in positions where they can interact with each other. For instance, intermolecular hydrogen bonds can be seen between NH3 molecules stand alone, between H2O molecules stand alone, or between NH3 and H2O
Molecules etc.,
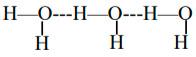
In simple terms, when a hydrogen atom bonded to an electronegative atom approaches a neighbouring electronegative atom, it creates intermolecular hydrogen bonding.
Let’s see the types of molecular forces in polymers to understand further the types of hydrogen bonding and branches of intermolecular hydrogen bonding.
Molecular Forces in Polymers
Atoms are held together in molecules by various types of bonds that depend on the valence electrons. By comparison, molecules are attracted towards each other by weaker bonds, which generally result from the electron configuration in the individual molecules. Thus, we have broadly two types of bonding:
1) Primary Bonds/Bonding due to Intramolecular forces
2) Secondary Bonds/Bonding due to Intermolecular forces
Secondary Bonds/Intermolecular Hydrogen Bonding
Secondary bonding is generally associated with the attraction between molecules, i.e. between two or more molecules, and is also known as intermolecular hydrogen bonding. Whereas primary bonds involve atom to atom attractive forces, secondary bonds involve attraction forces between molecules or intermolecular forces. In secondary bonding, the electrons are not transferred or shared which makes these bonds weaker than the primary bonds. There are three forms of secondary bonding – Dipole force, London force, and hydrogen bonding.
Dipole Force:
The types dipole and London forces are often referred to as Van Der Walls Forces, after the scientist who first studied and quantified them. Dipole forces arise in a molecule composed of two atoms that have equal and opposite electrical charges. Each molecule, therefore, forms a dipole, say, for hydrogen chloride; although the material is electrically neutral in its aggregate form, on a molecular scale, the individual dipoles attract one another, providing the proper orientation of positive and negative ends of the molecules of the substance. These dipole forces give a net intermolecular bonding within the material substance.
London Force:
London forces involve attractive forces between nonpolar molecules. That is, the atoms in the molecule do not form dipoles in the sense of the preceding paragraph. However, owing to the rapid motion of the electrons in orbit around the molecule, temporary dipoles form when more electrons happen to be on one side of the molecule than on the other side.
Hydrogen Bonding
Finally, hydrogen bonding occurs in molecules containing hydrogen atoms that are covalently bonded to another atom. Example: Oxygen in H2O
As the electrons needed to form a shell of the hydrogen atom are kept on one side of its nucleus, the opposite direction has a net positive charge that invites the electrons of the atoms in neighbouring molecules. Hydrogen bonding is generally a stronger intermolecular bonding mechanism than the other two forms of secondary bonding. It is important in the formation of many polymers.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





