The hydroxyl group is an important component of many significant molecules in organic chemistry. Learn about the hydroxyl group’s definition, structure, and polarity, as well as how it interacts with other functional groups in organic molecules.
On any given day, many people enjoy a glass of wine or use rubbing alcohol to disinfect a wound. And it’s unlikely that anyone on the planet does not consume sugar on a daily basis. However, it’s unlikely that these same folks are interested in the chemical composition of alcohol and sugar. We’ll learn about a critical component in the chemical constitution of sugars and alcohols in this session. While sugars and alcohols have a variety of chemical compositions, they all have two things in common: they are carbon-based and contain a pair of atoms known as the hydroxyl group.
Before delving into the details of hydroxyl groups, it’s necessary to first grasp the basics of carbon-based compounds. It’s called organic chemistry. These molecules, also known as organic molecules, are so vital to life that it would be impossible for it to exist without them. Organic substances such as DNA, proteins, and carbohydrates are essential for all living things. One of its most important building blocks is the hydroxyl group.
A phenol has –OH group(s) directly attached to carbon atom(s) of an aromatic system, whereas alcohol contains one or more hydroxyl (OH) group(s) directly attached to carbon atom(s) of an aliphatic system (CH3OH) (C6H5OH).
Hydroxyl Group
A functional group having connected oxygen and hydrogen atoms is known as a hydroxyl group. This functional group, which is also spelt hydroxy, is used by both alcohols and carboxylic acids. Alcohols are carbon chains with a side chain that contains a functional hydroxyl group. Because of the electronegativity of oxygen, alcohols have low polarity, allowing them to interact with other polar molecules such as water and certain solutes. Alcohols and carboxylic acids both have one or more hydroxyl groups in organic chemistry. An unbonded hydroxyl group can be found in both the negatively charged anion HO, also known as hydroxide, and the neutral radical HO•, also known as the hydroxyl radical. The name hydroxyl solely refers to the hydroxyl radical (OH) according to IUPAC definitions, whereas the functional group OH is called hydroxy group. Organic molecules with two or more hydroxyl groups, such as sugars and amino acids, become water-soluble when they include two or more hydroxyl groups.
Hydroxyl Group Structure and Formula
One hydrogen atom is linked to one oxygen atom to form a hydroxyl group. -OH or HO- are the chemical formulas for this compound. The hydroxyl group is linked to the carbon represented by the single bond.
Organic Molecules: Functional Groups
Let’s return to our basic understanding of an organic molecule to learn more about the characteristics of a hydroxyl group. Organic molecules are carbon-based and may also contain other elements such as oxygen, hydrogen, nitrogen, sulphur, and phosphorus. These molecules are made up of two primary sections structurally. The carbon backbone is the first stage, in which carbon atoms are bound together to form a carbon backbone.
The second is functional groups, which are tiny groupings of atoms connected to the carbon backbone, such as hydrogen and oxygen. The word “functional group” comes from the fact that it is the chemically reactive portion of the molecule.
The hydroxyl group is an example of a functional group (-OH). When hydroxyl groups are the dominant functional groups attached to carbon backbones, alcohols are generated.The hydroxyl group is on the far right of the chemical molecule ethanol’s structural formula (a type of alcohol). Methanol, isopropyl alcohol, and propanol are just a few examples of hydroxyl alcohols. hydroxyl groups are found in carbohydrate molecules, often known as sugars. Alcohols, on the other hand, lack another important functional group known as the carbonyl group (-CO). This feature distinguishes sugars from alcohols. As may be seen in the structure of a sugar called glucose, both examples include hydroxyl groups on both sides.
Properties of Hydroxyl Groups
Dissociation of water, alcohols, carboxylic acids, and a variety of other hydroxy-containing molecules is simple.Hydrogen bonding occurs in hydroxyl-containing compounds, causing them to stay together and result in greater boiling and melting temperatures than compounds without this functional group. Organic molecules with two or more hydroxyl groups, such as sugars and amino acids, become water-soluble when they include two or more hydroxyl groups.
Alcoholic hydroxyl group
Organic chemistry has long been a popular subject for scientists to research. The purpose of basic organic chemistry is to convey basic information about the organic molecules that surround us and to establish a solid foundation for future research into organic substances and the factors that control their behaviour. The organic compounds in a homologous series that have the same functional groups but differ by the presence of a –CH2 group. Alcohol is one of the many functional groups found in chemical compounds. This post will include alcohol structure, phenol structure, and other structures.
Can you explain what alcohol is?
Alcohols are chemical molecules in which the hydroxyl group replaces a hydrogen atom or an aliphatic carbon. As a result, an alcohol molecule is made up of two parts: one with the alkyl group and the other with the hydroxyl group. They have a pleasant aroma. They possess a unique set of physical and chemical properties. The presence of a hydroxyl group gives alcohols their physical and chemical characteristics. The structure of alcohol is influenced by a number of things.
Structure of alcohol
Alcohols are organic molecules assembled from carbon (C), oxygen (O), and hydrogen (H) atoms. When 2 carbons are present, the alcohol is called ethanol (also known as ethyl alcohol).The molecular formula of ethanol is C2H6O, indicating that ethanol contains two carbons and an oxygen. 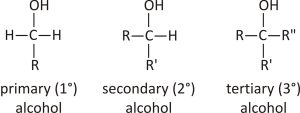
Alcohol classification
Alcohols are categorised into three categories based on the number of hydroxyl groups attached.
- Monohydric alcohols: Alcohols with one -OH groups.
- Dihydric alcohols: Alcohols with two -OH groups.
- Trihydric alcohols: Alcohols with three -OH groups.
An alcohol can be classified by the number of carbon atoms directly connected to the carbon that has a -OH group, depending on the number of carbon atoms directly attached.
- Primary alcohols: They have one carbon atom attached directly.
- Secondary alcohols: They have two carbon atoms attached directly.
- Tertiary alcohols: They have three carbon atoms attached directly.
Structure of phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula C6H5OH. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group 
Phenol classification
Phenols can be divided into three types based on the number of hydroxyl groups they contain.
- Monohydric phenols: Consist of only one -OH group.
- Dihydric phenols: Consist of only two -OH groups.
- Trihydric phenols: Consist of only three -OH groups.
Conclusion
Alcohols and phenols are characterised (i) by the amount of hydroxyl groups and (ii) by the hybridisation of the carbon atom to which the –OH group is connected, sp 3 or sp2. Alcohols, as non-aromatic compounds, have a hydroxyl group attached to the main chain, whereas phenols have a hydroxyl group directly bonded to the ring.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




