H-Bond is the formulation of the hydrogen bond, which is a type of attractive intermolecular force. This phenomenon occurs by the dipole-to-dipole interaction among hydrogen atoms and highly electronegative atoms. The hydrogen bond is generally weaker than the covalent or ionic bond. However, it is stronger than the Van der Waals forces.
Experts classified hydrogen bonds as a type of weak chemical bond. Since the hydrogen bonding is relatively stronger, it leads to a smaller number of interaction partners. For example, in the case of water molecules chemically represented as H2O, hydrogen is bonded covalently to the electronegative oxygen atom.
What is Hydrogen bonding?
With the simplest atomic structure, H2, hydrogen is the most abundant element in the universe and the third most abundant element on Earth. Hydrogen bonding is the formation of hydrogen bonds that are an attractive intermolecular force’s particular class. Hydrogen bonding is further classified into ‘Intermolecular’ and ‘Intramolecular’ hydrogen bonding.
- When hydrogen bonding happens between molecules that have either the same or other compounds, it is called Intermolecular hydrogen bonding. The most common examples are hydrogen bonding in alcohol, water, and ammonia.
- When hydrogen bonding happens within the molecule, it is called Intramolecular hydrogen bonding. It mainly occurs in compounds with two groups (one of the hydrogen atoms and the highly electronegative atom).
The Concept of Hydrogen Fluoride Bond
Hydrogen fluoride is a colourless, corrosive liquid or gas formed of fluorine and hydrogen atoms. Every time the hydrogen fluoride gets dissolved in running water, it is referred to as hydrofluoric acid. It is a raw material widely used to manufacture products such as gasoline, aluminium, and refrigerants.
Hydrofluoric acid is one of the most common acids used in almost all industries. This acid is so powerful that it can easily dissolve several compounds and substances such as oxides. The hydrofluoric acid’s acidity is mainly dependent on hydrogen-bond interactions of fluoride ions. In the gaseous state, hydrofluoric acid is highly poisonous.
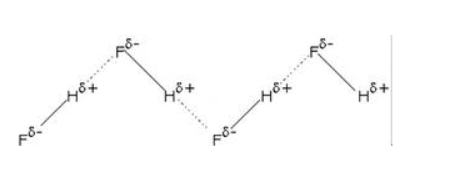
Hydrogen Bonding in Hydrogen Fluoride
Fluorine forms the strongest hydrogen bond featuring the highest electronegativity.
Hydrogen Fluoride Chemical Formula
HF is the chemical formula of hydrogen fluoride. This acid has the diatomic molecule along with strong intermolecular hydrogen bonds.
Formula: HF
Molar Mass: 20.006 g·mol−¹
Density: 1.15 g/mL
Melting Point: −83.6°C
Boiling Point: 19.5°C
Uses of Hydrogen Fluoride
There are many benefits and uses of hydrogen fluoride. Some of these are listed as follows:
- Industrial Uses
Hydrogen fluoride is vital in producing herbicides, refrigerants, kitchen products, pharmaceuticals, gasoline, plastics, electrical components, aluminium, and incandescent light bulbs. The compound is mainly used for enamel, etching glass, cleaning crystal, brass, and removing rust in industrial and laboratory settings.
- Commercial Uses
Since hydrogen fluoride has extremely corrosive qualities, diluted hydrofluoric acid is used commercially in automotive cleaners, stain removers, water-spot removers, and rust.
Why Does the Hydrogen-bond Compound Possess High Melting and Boiling Points?
Hydrogen-bonded compounds generally have a high boiling and melting point. These elevated temperatures of hydrogen-bonded compounds are accountable for the high energy requirement for breaking these bonds.
- When at room temperature, H2O is liquid. However, H2S, H2Se, and H2Te are in the gaseous state. Since hydrogen bonding gives rise to links in water molecules, it results in higher boiling and melting points than other chemicals.
- Since there is hydrogen bonding in NH3 and no hydrogen bonding in PH3, ammonia possesses a high boiling point.
- As ethanol has hydrogen bonds, its boiling point is higher than diethyl ether.
Conclusion
The hydrogen bonding with the hydrogen fluoride forms a strong hydrogen bond featuring the highest electronegativity. It is one of the most studied examples of hydrogen bonding.
The process of forming hydrogen bonds is an attractive intermolecular force’s special class. It arises because of the dipole-dipole interaction between the hydrogen atom and a highly electronegative atom. It mainly occurs between an electronegative atom and a hydrogen atom.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





