Introduction
In chemistry, hydrogen bonding can be defined as the process by which a hydrogen bond is formed. This process mainly occurs between an electronegative such as fluorine, chlorine, oxygen, and a hydrogen atom. The hydrogen bond is generally weaker than the covalent or ionic bond. However, it is stronger than the van der Waals forces. Experts classified hydrogen bonds as a type of weak chemical bond.
Today, on Hydrogen Bonding in Alcohols and Carboxylic acid, we will learn about hydrogen bonding in Alcohols and Carboxylic acid examples and related topics.
Hydrogen
Hydrogen is the first element of the periodic table. It is an odourless, tasteless and colourless gas under normal conditions. It is made up of diatomic molecules H2, and it is represented by the symbol H having the atomic number one. Hydrogen is made up of one electron and one proton.
Hydrogen bonding
As mentioned earlier, hydrogen bonding is the process where the hydrogen bonds are formed, which are an attractive intermolecular force’s special class that arises because of the dipole-dipole interaction between the hydrogen atom and highly electronegative atom. For example, in the case of water molecules, chemically denoted as H2O, hydrogen is bonded covalently to the electronegative oxygen atom. Further, hydrogen bonding is classified into two major sub-parts. These include:
- Intermolecular hydrogen bonding
Intermolecular hydrogen bonding can be defined as when hydrogen bonding occurs between different molecules with the same or other compounds. The most common examples are hydrogen bonding in alcohol, water, and ammonia.
- Intramolecular hydrogen bonding
Intramolecular hydrogen bonding can be defined as hydrogen bonding within the molecule only. It mainly occurs in compounds with two groups (one of the hydrogen atom and highly electronegative atom).
Alcohol
Alcohol is described as the organic compound where the hydroxyl group substitutes the hydrogen atom of an aliphatic carbon. Below we have discussed the physical and chemical properties of alcohol –
- Compared with other hydrocarbons, which have the same molecular masses, alcohol generally has a high boiling point.
- The hydroxyl group governs the alcohol solubility in the water.
- To form the corresponding alkoxide, the alcohol reacts with the active metals, including potassium, sodium, etc. These alcohol reactions indicate the acidic nature of the alcohol.
Carboxylic acid
Carboxylic acid is described as the organic compound which contains the carboxyl functional group. They mainly take place in nature and are manufactured by humans. During deprotonation, the carboxylic acid leaves the carboxylate anion. The chemical formula of carboxylate anion is R-COO– which is mainly used to form a wide variety of salts, including soaps. Carboxylic compounds can also be obtained from several routes, including lactic acid, citric acids, and fumaric acid, which are formed or produced through “fragmentation.”
Hydrogen bonding in alcohol and carboxylic acid
Alcohol is referred to as an organic molecule with an -OH group. Generally, in case any molecule that has a hydrogen atom is either linked to nitrogen or oxygen directly. Later, hydrogen bonding becomes easier. R is an alkyl or aryl group in the given diagram.
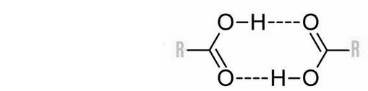
Why does the hydrogen-bonded compound possess high melting points and high boiling points?
The hydrogen-bonded compound generally has a high boiling and melting point. These elevated temperatures of the hydrogen-bonded compound are accountable for more energy required for breaking these bonds.
- When at room temperature, H2O is liquid. However, H2S, H2Se, and H2Te are found in the gaseous state. Since hydrogen bonding gives rise to links in water molecules, it results in higher boiling and melting points than other chemicals.
- Since there is hydrogen bonding in NH3; However, there is no hydrogen bonding in PH3; ammonia possesses a high boiling point.
- As ethanol has hydrogen bonds, its boiling point is higher than diethyl ether.
The conditions required for hydrogen bonding
In the molecule where the hydrogen atom is closely connected to the highly electronegative atom, the shared piece of an electron is attracted. As a result, the molecule’s end becomes slightly negative, whereas the other end becomes positive. Both the ends attract one another, which leads to the formation of a weak bond between them. This bond is referred to as the hydrogen bond. Here are two major conditions of hydrogen bonding –
- Each molecule should have a highly electronegative atom connected to the hydrogen atom. The more electronegativity, the higher the molecule’s polarisation.
- The electronegative atom size should be small. If the size is smaller, the electrostatic attraction will be more.
Conclusion
With this, we come to the end of the topic of hydrogen bonding in alcohols and carboxylic acid. In chemistry, hydrogen bonding plays a significant role as it is responsible for forming hydrogen bonds. Here we discussed alcohol and carboxylic acid in brief. Alcohol is the organic compound where the hydroxyl group substitutes the hydrogen atom of an aliphatic carbon. Carboxylic acid is the organic compound that contains the carboxyl functional group. They mainly take place in nature and are manufactured by humans.
The hydrogen bonding occurs in alcohol and carboxylic acid, which we studied in detail. At last, we also discussed the various conditions in which hydrogen bonding generally occurs. We hope this has helped attain a better understanding of hydrogen bonding in alcohols and carboxylic acid.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





