Water is an important mineral for our body, and 70% of it contains water. So, we need to keep ourselves hydrated all the time.
In this article, we will study concepts of hydration and hydrates. Hydrates are compounds and have various types.
Let’s begin by defining Hydration.
Hydration
Hydration is essential for our life. As we all know that our body depends on water for survival. All the parts of our body use water, whether it is tissue, cell, or organs, to work properly. For example, our body needs water to maintain the body’s temperature, lubricate our joints, and remove waste. Water is vital for our good health, and the importance of water makes hydration important.
If you face some problems staying hydrated, you can follow a few useful tips:
- Drink water when you stay outdoors for long to keep yourself hydrated.
- Add a slice of lime or lemon to water if you don’t like plain water.
- Always carry a water bottle with you.
- Drink water when you are feeling hungry. However, real hunger can’t be satisfied with water; it may only help postpone it. Water also helps in reducing weight.
You will become dehydrated if your body doesn’t get enough water. It will mean that you don’t have enough liquid in your body to function properly. Your urine may indicate it. If your urine is light yellow or colourless, you are sufficiently hydrated. If it is amber or dark yellow, it indicates dehydration.
There are also some other signs too that may be an indication of being dehydrated.
- Headache
- Extreme thirst
- Confusion
- Less or no urine
- Dry mouth
- Dizziness or lightheadedness
- Darker Urine than usual
- Fatigue
Hydrates
A hydrate is a compound that has absorbed water molecules from the environment in which it is kept and includes them in its structure. Organic, inorganic, and gas hydrates are the three types of hydrates. Here we have explained all three parts in detail.
Organic Hydrates – With the addition of water molecules in a carbonyl group of a ketone or an aldehyde, an organic hydrate is formed. In this hydrate, chemical reactions occur between the molecules of water and the compounds bonded with each other.
Gas Hydrates – Water molecules combine with the gas molecule in these hydrates. This usually happens around the methane gas. This hydrate is important as it may contain energy sources.
Inorganic Hydrates – In this type of hydrate, water molecules are bonded to the compound very loosely, and chemical reactions don’t occur with it. We can easily remove the water molecule or molecules from the compound through heating and other methods. When a hydrate loses its water molecules, it is called “anhydrous.” The most common hydrate is inorganic hydrates.
Naming system of hydrates
Some defined rules should be followed while naming and writing formulas of inorganic hydrates. Some examples of hydrate formulas are CaCl2 ⋅ 2H2O. The dot is not a symbol of multiply; it separates the two water molecules from the CaCl2, which indicates that the compound CaCl2 is not bonded to the water molecules, so it is a hydrate.
When giving a proper name to a hydrate, you first need to give the name of the salt and then prefix a name to the second part. The prefix is chosen based on the number of water molecules in the hydrate fixed by the salt. And this you will use the word hydrate to the prefix to give the complete hydrate name. For example, the hydrate name of CaCl2 ⋅ 2H2O is calcium chloride dihydrate. We have used the prefix “di” because it has two water molecules.
There are different prefixes for the different numbers of molecules of water. The table given below shows the prefix with the number of water molecules.
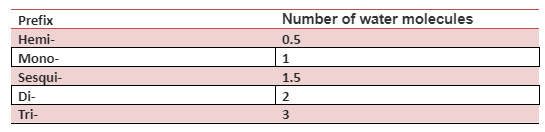
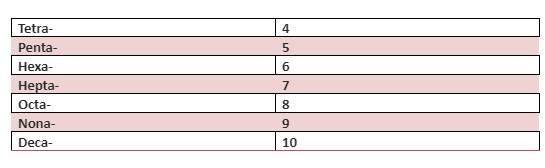
Conclusion
You will learn about hydration by reading these notes. By hydration, we mean the amount of water present in your body. It may be less or enough. If it is less, you may experience dehydration. The symptoms of recognising it and ‘how to keep the body hydrated all the time?’ are answered above. The article also discussed hydrates. Hydrates tell us about the number of water molecules. Hopefully, this hydration study material notes answers your queries about hydration.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





