Introduction:
Erich Huckel, a German chemist and physicist, published a theory in 1931 that could help to determine whether a planar ring molecule has aromatic properties. According to his rule, an
The aromatic molecule is cyclic and planar and possesses 4n+2 π electrons. Huckel’s Rule was named
after him.
Now,
Lets understand the definition of huckel’s rule, Huckel’s Rule is a series of methods for determining whether a molecule is aromatic, anti-aromatic,
or non-aromatic based on the number of electrons (N) and the physical configuration of the ring system. Within p orbitals, pi electrons are described as electrons that participate in pi bonds or lone pairs. A molecule must have a particular number of pi electrons within a closed loop of parallel, neighboring p orbitals to be aromatic. The pi-electron count is defined as a series of values formed by multiplying 4n+2 by any positive integer (i.e., n = 0, 1, 2, etc.). Huckel’s rule is the name of this rule. It’s used to determine whether a ring-shaped planar molecule or ion is aromatic. Six pi electrons (n = 1) are the most common instance, which may be found in benzene, pyrrole, furan, and pyridine.
Huckel’s rule equation
The rule can be applied to fully conjugated monocyclic hydrocarbons (also known as annulenes), as well as their cations and anions, to better understand their stability.
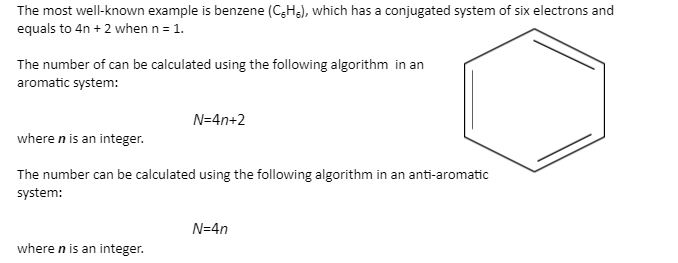
A compound is non-aromatic if it lacks a continuous ring of conjugated p orbitals in a planar conformation.
What is the 4n+2 rule?
When the total number of π electrons in a ring-shaped cyclic molecule can be equivalent to the formula “4n +2,” where n can be any integer with a positive value, the molecule must follow the Huckel rule (including zero).
Aromatic compounds are more stable than theoretical predictions based on hydrogenation data of simple alkenes; this extra stability is due to a delocalized cloud of electrons known as resonance energy.
- In a conjugated system of p o orbitals ( typically on sp2– hybridized atoms, but sometimes
sp-hybridized atoms), the molecule must have 4n + 2 (a so-called “Hückel number”)
electrons[7](2,6, 10,…)
- The molecule must be (almost) planar (the necessity for conjugation implies that the
p orbitals must be nearly parallel and able to interact).
- It must be a cyclic (rather than a linear) molecule.
- A continuous ring of p atomic orbitals must be formed in the molecule (there cannot be any
sp3 atoms in that ring, nor do exocyclic p orbitals count).
A compound is particularly stable if all of its bonding molecular orbitals are filled with paired electrons, according to Hückel’s Molecular Orbital Theory. Aromatic compounds are extremely stable in this regard.
When two electrons fill the lowest energy molecular orbital and four electrons fill each subsequent energy level (the number of succeeding energy levels is represented by n), all bonding orbitals are filled and no anti-bonding orbitals are occupied in aromatic compounds. This adds up to 4n+2 electrons in total. Benzene is a six-electron compound. It has 4 electrons left after the initial 2 electrons fill the lowest energy orbital. The orbitals of the next energy level are filled in by these four.
Abstract Knowledge: In this part, we will understand some abstract knowledge about the aromatic compound. Because of their negative impacts on the environment and human health, aromatic chemicals and ammonium are classified as priority pollutants. High amounts of these chemicals are frequently released into industrial and urban wastewaters. Various investigations have demonstrated that microbial consortia may remove aromatic chemicals and ammonium completely and simultaneously, however, the nitrification process can be impeded by inhibition, toxicity, and/or inactivation mechanisms under certain operating circumstances. Researchers have recently been able to find a wide variety of microbes capable of decomposing aromatic compounds present in culture media following recent developments in molecular biology.
Point To Remember:
- Huckle’s rule determines whether a planar ring molecule has aromatic properties.
- An aromatic molecule is cyclic and planar and possesses 4n+2 π electrons.
- A compound is non-aromatic if it lacks a continuous ring of conjugated p orbitals in a planar conformation.
- Benzene is an Aromatic Compound that possesses Aromaticity.
- Only the number of electrons in the conjugated system distinguishes aromatic and antiaromatic substances.
- An aromatic compound’s ring structure must be coplanar, which means that all of the atoms in the ring are in the same plane.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





