The Hofmann Elimination reaction was developed in 1851 by the famous German Chemist August Wilhelm von Hofmann. The Hofmann Elimination is also known as exhaustive Methylation. The Hofmann Elimination Reaction is based on the Hofmann Rule. Hofmann Rule emphasizes that the major product in reactions such as the Hofmann Elimination as well other Elimination reactions is the less stable Alkene. This rule is generally followed by most Elimination Reactions which follow a cyclic transition phase. The Hofmann Rule is sometimes also known as the Anti-Zaitsev Rule. This is because Zaitsev’s Rule states that the Elimination Reactions have a preference to yield a more stable Alkene as the product of the chemical reaction. This highly stable Alkene is called the Zaitsev Product. Whereas the Hofmann Rule states the opposite. Hofmann rule says that in the event of an Elimination Reaction, the less stable Alkene will be considered as the main product of the reaction as opposed to the more stable Alkene. This less stable Alkene is referred to as the Hofmann Product.
Step 1 of Hofmann Elimination-
This step basically implies that even amines can undergo elimination reactions. This step offers an explanation into what basically happens when silver oxide which is described by the formula Ag2O and Quaternary Ammonium salts are heated together in water. This step states that when this happens, the Quaternary Ammonium salts would undergo an Elimination Reaction which is called as an E2 Elimination So what basically happens in an E2 Elimination is that, 2 sp³ C atoms are converted into sp² C atoms by the process of this E2 Elimination Reaction. So, now moving further in this step, it says that if Amines are subjected to excess methyl iodide, they transform into the Quaternary Ammonium Salts. Next, the Silver Oxide (Ag2O) and the Water (H2O) will react with each other to form the Quaternary Ammonium Salts whereas the Silver Iodide will be left to precipitate. Now if we to express this step in terms of chemical reactions, we will do it as follows:-

Step 2 of Hofmann Elimination
In step 1 we saw the production of Quaternary Ammonium Salts using the Reaction of Silver Oxide and Water. We also saw that Silver Iodide precipitated as a result of that reaction. Now to continue from that, step 2 starts with the heating of a hydroxide which will give rise to 1, 2- or β elimination which is in turn promoted by a base. This reaction produces an amine and an alkene. Now in this step, there is an interesting part. The regioselectivity in this reaction step is in reverse to that predicted by Zaitsev’s Rule. Zaitsev’s Rule predicts that the eliminations have a tendency to produce the more stable alkene as opposed to the less stable alkene as the end product. The more stable alkene is known as the Zaitsev product whereas the less stable alkene is known as the Hofmann Product. The Hofmann Product is also known as the Anti-Zaitsev Product. The figure below offers an in-depth description of the operations performed in Step 2.
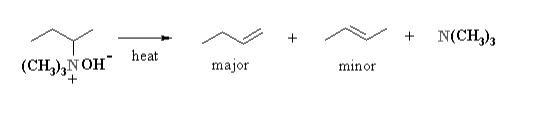
Step 3 of Hofmann Elimination
In Step 2 we saw how during the chemical reaction, an alkene and an amine were produced. We considered the less stable Alkene as the main product of the reaction since this chemical reaction follows the Hoffmann Rule. The outcome of The Hofmann Elimination Reaction is defined by the steric effects of the large leaving group and the alkyl chain. NH2– and NR2– are very poor leaving groups (both anionic), but NR3 is much better (neutral). Compare this with -OH and H2O in the dehydration of alcohols.
Uses of Hofmann Elimination
Since The Hofmann Elimination produces a less-stable alkene, Alkene is used to produce many end products like Alkanes and Alkynes. The main application of the Hofmann Elimination Reaction is in the Pharmaceutical Field to produce a lot of different end products. Among others, The Hofmann Elimination Reaction plays a very important part in the production of anthranilic acid, the production of sweetening agents, and most importantly, the production of Benzene.
Conclusion
We have described The Hofmann Elimination Reaction briefly. In the Introduction topic, we first looked at a brief history of The Hofmann Elimination and then we proceeded to explain this reaction in detail.
Then we explained the first step of The Hofmann Elimination in detail. In the first step, we saw what happens when Silver Oxide and Quaternary Ammonium Salts are heated together in the water. In step 2, we further moved on with the reaction and we saw that an amine and an alkene were produced. We observed that due to The Hofmann Rule, the less stable Alkene was considered the main product of this reaction.
Then we saw the third and final step of The Hofmann Elimination Reaction. After that, we saw quite a few important uses of this reaction in our day-to-day lives. We saw that this reaction is very essential to the production of very popular chemicals such as Benzene.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





