Introduction:
According to Charles Coulston Gillispie, John Dalton “It is assumed that in the vapour phase, the separation of gas particles from one another bears a modest whole number relation to their interatomic distance in solution. If this ratio is constant for each gas at a given temperature, Henry’s law follows as a result.”
The depth-dependent dissolution of oxygen and nitrogen in the blood of underwater divers that alters during decompression, resulting in decompression sickness, is an example of Henry’s law in action. One’s encounter with carbonated soft drinks, which contain dissolved carbon dioxide, is a daily example. The gas above the drink in its container is practically pure carbon dioxide before it is opened, and it is at a pressure higher than atmospheric pressure.This gas escapes when the container is opened, lowering the partial pressure of carbon dioxide above the liquid and causing degassing when the dissolved carbon dioxide leaves the solution.
Henry’s law Formula:
The following equation can be used to express Henry’s law:
C=kPgas
The solubility of the gas in a particular solvent at a given temperature is denoted by C. In addition, the units are either M or gas/L. Henry’s law constant is represented by the symbol k, which is a constant. Furthermore, Pgas denotes the gas’s partial pressure.

Constants of Henry’s Law in Different Forms:
The proportionality constant of Henry’s law can be defined in a variety of ways. These paths can be divided into two basic categories. To begin, one option is to use the aqueous phase as the numerator and the gaseous phase as the denominator (“aq/gas”). This gives us the solubility constant H of Henry’s law. Furthermore, as the solubility of the substance grows, so does its value. Alternatively, the numerator and denominator can be switched (“gas/aq”), resulting in the KH Henry’s law volatility constant. In addition, when the solubility increases, the value of KH drops.
The following factors influence the value of a Henry’s law constant of a gas:
- The gas’s composition
- The solvent’s composition
- Pressure and temperature
As a result, different gases in different solvents have different Henry’s laws constants, as seen visually below.
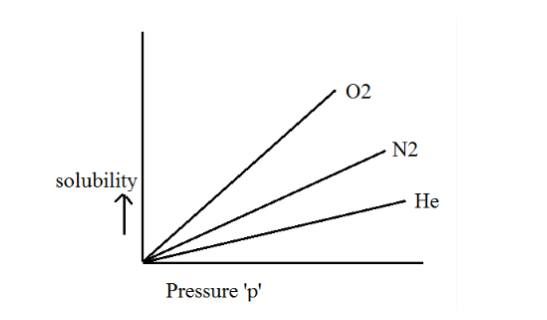
Henry’s Law’s Limitations:
- This law applies only when the system’s molecules are in a condition of equilibrium.
- When gases are subjected to extremely high pressures, Henry’s law does not apply.
- When the gas and the solution are involved in chemical reactions with each other, the law does not apply.
Henry’s law in Geochemistry:
In the field of geochemistry, Henry’s law is quite useful. This law can be used to calculate the concentration of gas in mines. Furthermore, when the solution is prepared near the infinite dilution, Henry’s law aids in the computation of a wide variety of solutes. This law is also used by environmental chemists to calculate the concentration of gases in lakes, oceans, and other bodies of water.
Henry’s law is a rough approximation that works for dilute solutions. Furthermore, the further a system deviates from ideal solutions, the less accurate the calculation will be. Furthermore, when the solvent and the solute are chemically similar, this law works best.
Applications of Henry’s Law:
- In the manufacture of carbonated beverages
CO2 solubility increases under high pressure. Solubility declines as the bottle is opened to air pressure, and gas bubbles are expelled from the liquid.
- Climbers and those who live at high altitude will benefit from this.
Hypoxia is a situation in which the concentration of oxygen in the blood and tissues is so low that the person feels weak and unable to think clearly.
- When it comes to scuba diving,
Because of the hydrostatic pressure, gas is breathed at ambient pressure, which increases with depth. According to Henry’s law, the solubility of gases increases with depth, therefore body tissues take on more gas with time until they are saturated for the depth, and vice versa.When a diver ascends, he or she is decompressed, which reduces the solubility of the gases dissolved in the tissues. Bubbles may form and grow if the supersaturation is too high, and the presence of these bubbles can create blockages in capillaries or distortion in the more solid tissues, resulting in decompression sickness. To avoid harm, the diver must ascend slowly enough for the excess dissolved gas to be taken away by the blood and discharged into the lung gas.
Conclusion:
Henry’s law is simply a rough approximation that can be used with dilute solutions. The computation will be less precise the further a system deviates from ideal solutions (as with any gas law). In general, Henry’s law is most effective when the solute and solvent are chemically comparable.
Henry’s law is employed in real-life situations.Hence,the law calibrates with various situations be it scientific or day to day routine.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





