Michael Faraday first discovered benzene in 1825, which showed that it was a liquid type of colourless liquid with its molecular formula, or C6H6. This formula characterises its organic compound to be highly unsaturated and reactive. It is the opposite of benzene alkanes, as it does not participate in oxidation and reduction reactions. Due to the formation of double bonds with benzene carbon, other reagents like HCl do not participate in the reaction. Substitution reactions are also often observed in benzene. We will find that it has the property of converting more than one hydrogen atom into a radical. Benzene is mainly an aromatic compound; like other compounds, it is also described based on structure and chemical reactivity, as we will find in chemistry.
Significance of Halogenation
According to known information, more than one halogen atom occurs during the halogenation reaction. Halogens are elements such as iodine, chlorine, fluorine, and bromine, which have their properties. They also present normal behaviour in some circumstances, which we will look at next.
Types of Halogenation
In chemistry, we study both organic and inorganic compounds for reactions; elements of the halogen group are also part of such reactions, which can be of many types. These reactions can be divided into many parts depending on their substrate. It can also be divided into, which are as follows.
- Reaction with saturated hydrocarbons
- Reaction with unsaturated organic
- Reaction with electrophilic substitution
- Free radical halogenation
- Reaction with alkenes and alkynes
- Reaction with Aromatic Compounds
Halogen Addition- This reaction will expose the halogen atom to the unsaturated carbon, its main component, where compounds such as alkynes and alkanes are commonly seen. During these reactions, it combines with halogens. In this type of reaction, we often see elements like bromine, chlorine, and iodine react with ethane, some of which are as follows.
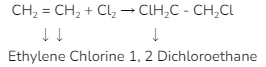
Halogen Substitution – In this reaction, we will look at the reaction with saturated hydrocarbons, where the halogen atoms replace hydrogen atoms during the reaction. Hydrocarbons are mainly treated with free radical halogens. We can also easily see the interaction of saturated hydrocarbons with halogens, which would mainly indicate the presence of C–H bonds during the reaction of alkanes with alkanes halogens. We will find one thing in the example taken here. When halogens react with alkanes, we get alkyl halides, but the effect of heat is mainly seen in this reaction.
CH4 + Cl2 +Heat CH3Cl + HCl
Methyl chloride
CH3CH3 + Br2 + Heat CH3CH2Br + HBr
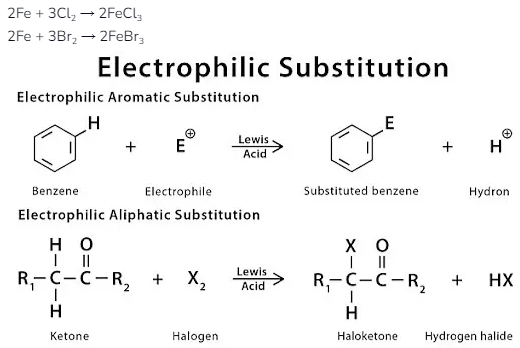
Similarly, for other substitution reactions or electrophilic halogenation in the presence of Lewis acids, the reactions are something like this:
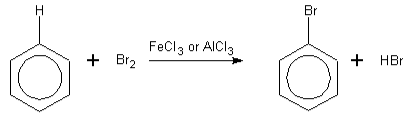
Electrophilic Substitution Reaction- Electrophilic substitution reaction, which we do only with aromatic compounds of halogens, in which mainly compounds such as bromine and chlorine participate. To complete this reaction, the work is done in the presence of Lewis acid. This is a kind of laboratory method, where apart from the halogen-halogen bond, their polarisation can also be seen. After which, the halogen molecules become more electrophilic after the reaction. The reaction of benzene with bromine and chlorine is to be seen here, where an electrophilic substitution reaction takes place, in which the presence of the catalyst predominates. This catalyst is used in the form of chloride or iron, which helps produce the product in the reaction.
Hunsdiecker reaction- In the Hunsdicker reaction, the carboxylic acid is the main reactant, which helps to provide the chain shortened halide product during the reaction. Apart from this, the carboxylic acid present here converts to silver salt, where oxidation of halogen compounds is also seen.
RCO2Ag + Cl2 → RCl + CO2 + AgCl
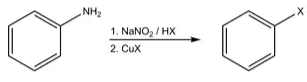
Sandmeyer reaction- It is also the site of the Sandmeyer reaction, which is used to produce products such as diazonium salts aryl halides, from which aniline is also obtained.
Conclusion
We study both organic and inorganic compounds for reactions; elements of the halogen group are also part of such reactions, which can be of many types. These reactions can be divided into many parts depending on their substrate. A halogenation reaction occurs when one or more fluorine, chlorine, bromine, or iodine atoms replace hydrogen atoms in an organic molecule. Fluorine > chlorine > bromine > iodine is the sequence of reactivity. Fluorine is a particularly aggressive element capable of severe reactions with organic compounds.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





