Graphite, also called the black lead, is a crystalline form of carbon, typically occurring in metamorphic rocks as flakes or crystalline layers. Graphite is formed due to the metamorphosis of carbonaceous sediments. In nature, it is also found in igneous rocks, meteorites, and the mineral plumbago. It is a covalent solid whose constituent carbon atoms are held firmly by covalent bonds. Usually, covalent solids are hard and strong. However, black lead is soft due to its structure. It is the most stable form of carbon, and when subjected to high pressure and temperature, it can turn into a diamond, but it takes millions of years.
The principal producer of black lead worldwide is China, followed by Mexico and Canada. The black lead reserves of China are the second-largest worldwide, the first being that of Turkey. This black lead is found in Jammu and Kashmir, Rajasthan, Orissa, West Bengal, and Bihar in India.
Overview: Introduction to Graphite Structure
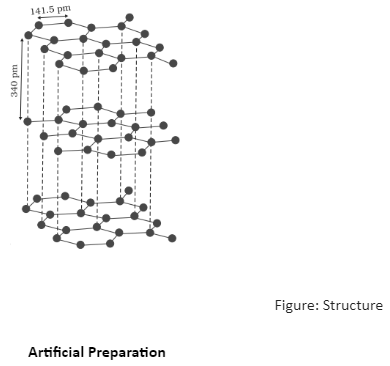
Graphite has a two-dimensional planar structure. Each carbon in it is sp2 hybridised. Each individual carbon atom bonds to three of its neighbouring carbon atoms through covalent bonds, forming hexagonal planer rings in a single layer. Each atom’s last free valence electron is free to move between different layers. The Carbon-Carbon covalent bond length is 141.5 pm in the rings, indicating strong bonding. Thus, black lead has two-dimensional sheet-like polymeric rings. Each sheet or layer may be regarded as a fused system of benzene rings. Any two successive sheets are about 340 pm apart. This significant distance between two successive layers does not permit the formation of covalent bonds. Successive layers can slide one over the other and are held together by weak van der Waals forces.
Artificial Preparation
Graphite in its pure form can be prepared artificially by heating powdered coke mixed with little sand and Iron (III) oxide in an electric furnace to a temperature of 3000 ℃

It can be made using sucrose, sulfuric acid, and sodium bicarbonate in the laboratory.
Chemical and Physical Properties
In appearance, Graphite is a greyish black, opaque substance with a metallic lustre. It marks a black stain on the paper. Its structure, as discussed above, can be used to explain its characteristic properties as follows:
- Slippery nature – Weak van der Waals’ forces bond the successive layers in Graphite. It permits the sliding of one layer over another. This also allows the cleavage of the structure along the lines of planes. Consequently, black lead is soft and slippery and can be used as a lubricant.
- High melting point – The carbon atoms in each layer are bonded by strong covalent bonds, due to which the melting point is high, about 3500℃
- Conductivity – Each carbon in Graphite is sp2 hybridised. Thus, one valence electron of each carbon atom is free to move from one point to another. The unhybridized orbitals containing one electron each overlap laterally to form bonds in the same layer. These delocalised electrons are free to move under the influence of heat and electric fields. Thus, it makes a good heat and electricity conductor.
- Low density – Its density is 2.26 g/cm3, which is higher than diamond due to the considerable distance between successive layers.
- The chemical bonds in Graphite are stronger than those that make up a diamond. Black lead is also highly resistant to chemical attacks and self-lubricating.
- It burns in the air at 700℃ to form carbon dioxide.
In bulk form, it is non-flammable but combustible.
- Graphite does not rapidly react with water or air. It has a violent reaction with fluorine, chlorine dioxide, and potassium peroxide, powerful oxidising agents.
Graphene
Graphene is an allotrope of carbon formed of a single layer of Graphite, the ‘mother material’. It comprises a layer of sp2 hybridised carbon atoms covalently bonded in a hexagonal lattice structure. Graphene’s properties exceed its mother material as it is the strongest material in the known universe, forty times stronger than diamond, excellent heat and electricity conductor. In 2003, it was discovered that Graphene has promising applications in nanotechnology, biotech, optic sensors, and other uses.
Uses
- With petroleum jelly to form black lead grease, a lubricant.
- For making the electrodes of electric furnaces.
- For making crucibles for melting metals due to its high melting point.
- For making carbon brushes for electric motors
- For making pencil leads because it can mark black on paper (pencil leads are made by mixing black lead with variable quantities of clay).
- It is used in nuclear reactors as moderators to slow down the speed of neutrons.
- It is used to make Graphene, the strongest material in the known universe, with many promising nanotechnology and biomedical applications.
- As the standard state for defining the heat formation of carbon compounds, it generally finds use in thermochemistry.
Conclusion
Graphite, or black lead, is a greyish black opaque covalent crystalline solid made of carbon. It is found naturally between metamorphic rocks and can be artificially prepared using sand and powdered coke. It has a two-dimensional structure, with covalently-bonded carbon atoms in hexagonal planar rings forming its layers, and weak van der Waals forces keep the adjacent layers together.
Due to its structure, black lead is soft and slippery, has a high melting point, is a good conductor of heat and electricity, has high chemical resistance, and has low density. Examples of graphite are lubricant, pencil leads, the electrode for electrical furnaces and the most promising, strongest material in the universe, graphene.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





