Charles’ law, a gas law, states that in ideal gas mixtures, the pressure is inversely proportional to the volume of a gas. The law takes its name after the French scientist, Jacques Charles Lilienfeld.
Charles’ law is important in several fields of science, including astronomy, aviation, chemistry, and chemistry. To satisfy Charles’ law, the density of the gas must be constant.
Let us take a closer look at Charles’ law and graphical representation Of Charles’ Law.
Charles’ Law
According to Charles’ law, in a closed system, the volume of a gas is directly proportional to its temperature (in Kelvin). As a result, this law describes the link between the gas’s temperature and volume. We can use the following formula to express Charles’ law.
V ∝ T
Where,
V = volume of gas
T = temperature of the gas in Kelvin
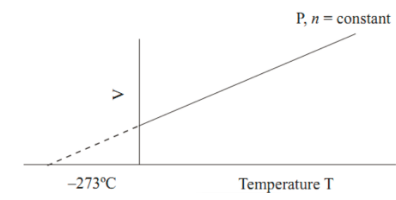
Graphical Representation of Charles’ Law
Mathematical Expression of Charles’ Law
V1 / T1 = V2 / T2
Furthermore,
V1 / T1 = V2 / T2 ⇒ V/T = constant= K2
So,
V= K2T
As a result, the gas’s pressure, volume, and unit of volume determine the value of K2.
Charles’ Law: Discovery and Naming
The law’s name is a tribute to the French mathematician, physicist, inventor, and balloonist Jacques Alexandre César Charles.
French chemist and physicist Joseph Louis Gay-Lussac wrote a paper outlining how gases expand when heated. He published it just a few days after the first human-crewed hot air balloon flight in 1802.
In 1787, Charles experimented with filling five identical containers with various gases. After that, he raised the temperature of the containers to 80 degrees Celsius. He then saw that all gases had grown in volume by the same amount. Charles deduced that the volume of gas increases linearly with the absolute temperature of that gas under constant pressure
Gay-Lussac mentioned this experiment in his paper, attributing it to Jacques Charles’ unpublished work from the 1780s. That is why in honour of Charles, Gay-Lussac titled the statute ‘Charles Law.’
Application of Charles’ Law in Real Life
This law has several applications in everyday life.
Inflated Items
Suppose you are outside on a cold day with a balloon. You will notice that the balloon crumbles. On the other hand, the balloon regains its shape when placed in a warm environment. What causes this to happen?
This phenomenon occurs because the volume reduces when the temperature drops on a chilly day. Now, according to Charles’ Law, the temperature rises as soon as you enter a heated room. Likewise, as the temperature rises, the volume rises as well. As a result, the balloon returns to its former shape.
This behaviour is true of any inflated item. That is why you should check the pressure in your automobile tires while going outside on chilly days.
Baking
Our kitchens are no exception to Charles’ Law. Have you ever tried your hand at baking?
Yes?
Then, you are probably familiar with the most popular ingredient in the process: yeast. Baking yeast is commonly used to generate fluffy baked items. It induces the release of carbon dioxide bubbles.
When the temperature rises, the carbon dioxide bubbles expand, acting as a leavening agent, causing the bread items to puff up. With a higher temperature, the carbon dioxide bubbles will expand even more.
Hot Air Balloons
You may have been curious about how a hot air balloon works.
Temperature and volume are precisely proportional to each other, according to Charles’ Law. When you heat a gas, its density decreases, and it expands. Warm air is less dense than cold air, which is lighter. Warm air also has less mass per unit volume. That is why warm air rises above cold air.
The hot air balloon uses the same principle. The air inside the balloon is heated, causing its temperature to increase compared to the surrounding air. And the balloon rises up.
Pop-Up Turkey Timer
Charles’ rule also governs the operation of the Pop-Up Turkey Timer (Thermometer). Let us look at how it does so!
If you recall, Charles’ law says that gases expand when heated. The Pop-Up Turkey Timer works on the same concept. When you roast your turkey in an oven, you insert the tool inside the turkey. The gas inside the thermometer expands as the temperature rises. When the timer beeps, it is time to remove the turkey from the oven.
Conclusion
This graphical representation of Charles’ Law notes discusses the law and its history. It also discusses the applications of Charles’ Law. In this gas law, we consider the density of the gas as constant. We also plotted the law on the graph.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





