Glucose is a monosaccharide that our body receives through food and utilizes as an important source of energy. The fundamental molecular indication of glucose is C6H12O6.
The process by which carbon dioxide is converted into glucose in plants is known as the Calvin cycle. In this process, the formation of one glucose molecule requires 18 ATP and 12 NADPH. In plants, glucose is mainly found in fruits and in the different other parts. The best example of the use of glucose is a symbiotic relationship between plants and fungi, in which fungi provide water and minerals to the plants, and in turn, plants give glucose to the fungi as a source of energy.
Properties of Glucose
The properties of glucose are:
- The molecular weight or molar mass of glucose is 180.16g/mol.
- The density of glucose is 1.54g/cm3.
- The melting point of glucose is 146°C
- It is a simple sugar that is a monosaccharide.
- Glucose is also called dextrose.
How Glucose is Processed by Our Body
Glucose enters our body as galactose and fructose in various forms, which are mainly isomers and monosaccharides of glucose. Our body breaks down complex sugars into galactose, glucose and fructose for soaking up and metabolism.
In addition to obtaining glucose from food, our body prepares glucose by a procedure called gluconeogenesis. Gluconeogenesis is the formation of glucose with the help of non-carbohydrate compounds. It is a ubiquitous process present in plants, animals, fungi, bacteria and other microorganisms.
Glucose is stored in the form of glycogen in our muscles and liver. Whenever there is a deficiency of glucose in our body, this stored form of glycogen gets converted into glucose and then released into the blood. The whole process is carried out with the help of the hormone glucagon, which is responsible for the increase in blood glucose levels. This hormone is synthesized by the alpha cells of islets of Langerhans (it is the endocrine part of the pancreas). With the help of glucose and oxygen, our body makes the energy currency of the cell known as ATP (Adenosine Triphosphate).
Diseases Associated with Blood Glucose Level
The diseases associated with glucose often occur when blood glucose levels are either too high or too low. Blood glucose level is maintained by two hormones:
- Glucagon
- Insulin
Diseases
Hyperglycemia: This is a condition where blood sugar level gets higher than normal. The symptoms of hyperglycemia are:
- Fruity-smelling breath
- Nausea and vomiting
- Shortness of breath
- Weakness
- Dry mouth
When the blood glucose level gets higher than normal, it leads to diabetes mellitus. In this condition, glucose gets released out of the body through the urine; such a condition is called glycosuria. Ninety-Nine per cent of glucose from the filtrate is reabsorbed by the proximal convoluted tubule of the kidney.
Hypoglycemia: This is the condition where blood glucose level gets lower than normal. The symptoms of hypoglycemia are:
- Irregular or fast heartbeats
- Fatigue
- Pale skin
- Shakiness
- Anxiety
- Sweating, hunger, and irritability
Lab Test for Determining Blood Glucose Level in the Body
Normal blood glucose level of the body ranges from 70 to 90 mg/dL. Higher ranges than this lead to prediabetes or diabetes. The test used for the determination of blood glucose level is the A1c test. This test is useful for getting the exact information of the blood glucose in your body. This A1c test is mainly used in the diagnosis of diabetes and prediabetes. It is considered a preliminary test in the management of diabetes.
Formation of Cyclic Sugars Like Glucose
Sugars are mainly polyhydroxylated (containing a large number of OH groups) chains of carbon atoms that, in addition, feature an aldehyde or ketone group. In general, all sugars have the 1:2:1 ratio as Carbon: Hydrogen: Oxygen. Glucose possesses the property of undergoing inter or intramolecular reactions between the groups like hydroxyl and carbonyl.
Cyclization Reaction With D-glucose
Reaction:
It is an intramolecular reaction when an aldose directly cyclizes the hydroxyl group present on the C5. Due to such conditions, C5 shows an intramolecular reaction with the C1 carbonyl group of the aldehyde. The product is hemiacetal.
Fischer Projection
It is the two-dimensional representation of sugar D-glucose on the left-hand side called Fischer Projection.
Haworth Projection
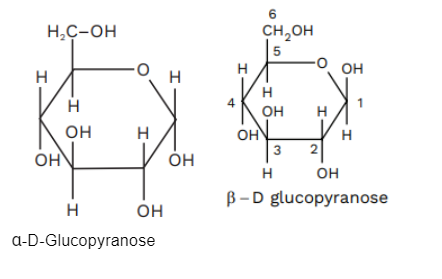
The resultant 3-dimensional representation of D-glucose cyclization product hemiacetal is called Haworth Projection.
Important things to remember
- The structures produced through such reactions are cyclic due to the closed ring, which is produced by strong chemical bond formation.
- This attack on aldehyde to form hemiacetal results in a 6-membered ring structure.
- The 6-membered rings are called aldose and the 5-membered rings are called ketone or furanose.
- The prefix D is used to show the direction of the optical isomer (a compound with asymmetric groups surrounding a central atom)
- This rotates the plane-polarized light.
- D isomers have the OH of the chiral C5 and L isomers have the OH of the chiral C5 pointing to the left.
D-Glucose
This is the most commonly occurring simple sugar. It acts as an important base or building block for the formation of disaccharides, sucrose, lactose, higher oligosaccharides and polysaccharides. It is a sugar unit in cellulose and starch. D-glucose is also present in open form, but 99% of it is present in the form of a solution.
Conclusion
Glucose is a simple sugar, which acts as a building block for disaccharides, oligosaccharides, and many polysaccharides. Glucose is also called hexose sugar, as it consists of six carbons. Extensive information about glucose is available as numerous researches are conducted on glucose, the most popular being D-Glucose structure. Researchers have been awarded for their work on educating about the different structures of glucose like D-glucose and other sugars.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





