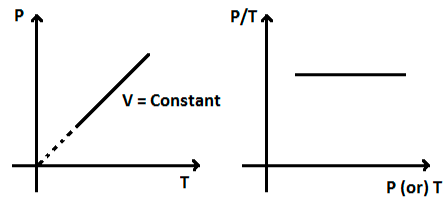
In Gay-Lussac’s Law, the volume remains constant, whereas the pressure remains directly proportional to the temperature. The usual equations for Gay-Lussac’s law are
P/T = constant or Pi/Ti = Pf/Tf.
Where,
Pi and Ti are the initial pressure and absolute temperatures.
Pf and Tf are the final pressure and absolute temperature.
Gay-Lussac Law
In Gay-Lussac’s Law, the volume remains constant, whereas the pressure remains directly proportional to the temperature. The Law states that when the temperature rises, pressure rises as well. The gas molecules’ kinetic energy increases as the temperature rises, causing this phenomenon. Because of the increased energy, molecules hit the container’s walls with more force, resulting in higher pressure.
Amonton’s Law is another name for Gay Lussac’s Law. Amonton demonstrated the same logic by inventing the thermometer with a current temperature reading.
History
In 1802 a French scientist and physicist named Joseph Louis Gay-Lussac found that if you keep the volume of a gas constant (such as in a closed container) and apply heat, the pressure of the gas will rise. This is due to the gases’ higher kinetic energy, which causes molecules to collide more forcefully with the container’s walls (resulting in greater pressure).
Gay-Lussac’s Law Example
You may notice a low tire pressure indicator on your automobile as the temperature decreases in the winter. This phenomenon happens because of the relationship we see in Gay Lussac’s Law. Because temperature and tire pressure are closely linked with each other, when the temperature lowers, the amount of pressure in the tire drops as well. The overall volume and mass of gas within, on the other hand, remain unchanged. (The tire volume changes with significant temperature swings, while it remains roughly constant with moderate temperature variations.).
The same may be said about propane tanks. Depending on the temperature, the pressure in the tank may be lower or greater. The pressure gauge on the tank will read higher as the temperature rises.
Watch or attempt the collapsing can experiment to see how pressure falls as temperature drops. This experiment isn’t a perfect representation of Gay Lussac’s Law, but it is a fair example of temperature-induced pressure fluctuations. Gay Lussac used a hard container with a predetermined capacity in his tests.
The item you wish to cook is submerged in water inside a pressure cooker. Water vapour is created as the temperature of liquid water rises. Because the vapour cannot exit the pressure cooker, the volume does not change. The water vapour pressure continues to rise until the temperature of the water, and water vapour exceeds the typical boiling point of water (100 °C). Food may be cooked significantly faster at this higher temperature. When tough meat is cooked in a pressure cooker, it becomes considerably softer.
Did you know that the air pressure within the tires changes as you drive a car? The air pressure in a car’s tires rises after driving. The air inside the tires heats up due to friction (a contact force) between the tires and the road. Because the tires are essentially a fixed-volume container, the air cannot expand; hence the pressure raises — Gay Lussac’s Law.
Importance of Gay-Lussac’s Law
This gas rule is important because it demonstrates that raising the temperature of a gas causes its pressure to rise proportionally (provided the volume remains constant). Similarly, as the temperature drops, the pressure drops proportionally as well.
Conclusion
When solving a Gay-Lussac’s Law problem, keep the following factors in mind:
- The volume of gas is kept constant.
- As the temperature of the gas rises, so does the pressure.
- When the temperature drops, so will the pressure.
The kinetic energy of gas molecules is measured by temperature. At a low temperature, molecules move more slowly and are less likely to collide with the container wall. The molecules’ velocity increases as the temperature rises, impacting the container’s walls more often, increasing pressure. The direct relationship only applies if the temperature is in Kelvin. The most typical error one makes while solving this problem is neglecting to convert to Kelvin or incorrectly converting. Another blunder is failing to include crucial figures in the response. Use the fewest significant numbers specified in the problem.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





