Inductive effects mean forming of a permanent dipole due to unequal sharing of bonding electrons in the molecule. This effect can arise in sigma bonds, whereas the electromeric effect can arise in pi bonds. This article talks about gauging the acidity or basicity of a compound with the inductive effect.
Effect of inductive effect on acidity and basicity of a molecule
We can estimate the acidity and basicity of complexes using the inductive effect. In general, electron-withdrawing groups (EWG) increase the acidity of a complex, while electron-donating groups (EDG) reduce the acidity of a compound.
- This is because the conjugate base of the acid, RCOO–, is stabilised via delocalisation of the formed negative charge if R is electron-withdrawing.
- If R had donated electrons, the conjugate base would be destabilised due to interelectronic repulsions.
- Therefore it can be given that the +I effect decreases the acidity, and the -I effect increases the acidity.
- For example, formic acid is more acidic than acetic acid (CH3COOH) because it inherits the +I inductive effect of the methyl group attached to the carboxyl group.
Types of inductive effect
-I Effect
When a particle or group draws bonding electrons toward it and obtains a partial negative charge, it is referred to as a -I group and the relating effect is referred to as the electron-withdrawing inductive effect, or the -I effect.
+I Effect
When a particle or group donates electrons to a bond and gains a partial positive charge, it is referred to as a +I group, and the related effect is referred to as the positive Inductive Effect or the +I effect.
Gauging the acidity or basicity of a compound with the inductive effect (solved example) questions
Question 1: What among the fluoro acetic acid and chloroacetic acid has greater acidity?
Solution: Since the electronegativity of fluorine and chlorine is 4.1 and 2.8, respectively, it is expected that Fluoro acetic acid is stronger. The pKa values of these two acids are 2.39 and 2.68, respectively. Indeed, fluoro acetic acid is stronger, but the difference is not huge.
Question 2: The comparative basic strength of amines is unaffected by __________ .
- a) Inductive effect
- b) Mesomeric effect
- c) Steric effect
- d) Stabilisation of cation by hydration
Solution: The correct answer is b. The relative basic strength of amines does not depend upon the mesomeric effect. This effect is used qualitatively and describes the electron-withdrawing or releasing properties of substituents based on relevant resonance structures.
Question 3:
Arrange the groups mentioned below according to decreasing -I effect.
- CN > F > Br > Cl > COOH > I > H
- CN > COOH > F > Cl > Br > I > H
- H > COOH > CN > I > Cl > F > Cl
- COOH > CN > F > Br > Cl > I > H
- None of these
Solution: Option b is the correct answer.
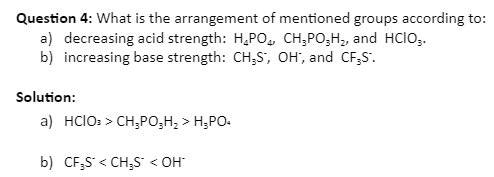
Inductive effects and charge delocalization have a significant impact on a compound’s acidity or basicity. A molecule’s acid-base strength is strongly influenced by its structure.
If A–H or B–H+ bond is weaker, then it is more likely it will dissociate into an H+ ion. Furthermore, any factor that favours the dissociation of H+ favours the stability of the lone pair that is present on the conjugate base, which makes the conjugate acid a stronger acid.
Parts of a molecule like atoms or groups of atoms play a role in influencing acid or base strength through inductive effect, which reduces the strength of an O–H bond and makes hydrogen break free more easily as H+ ions.
Conclusion
This article explains gauging the acidity or basicity of a compound with the inductive effect. The inductive effect is a partial shifting of bonds of σ electrons towards the electronegative element of the bond. This effect can arise in sigma bonds, whereas the electromeric effect can arise in pi bonds. In this article, we have studied gauging the acidity or basicity of a compound with the inductive effect using solved examples.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





