Introduction
An atom is a complex structure, with protons and neutrons inside a nucleus and electrons revolving outside. Studying the atomic structure helps in understanding the simple and profound concepts of bonding and chemical reactions in chemistry.
- The idea of the atomic structure dates beyond the period of Democritus
- Democritus discovered that all matter and living things in the universe are made up of tiny atoms
- Further study of the atomic structure started with John Dalton
The discovery of atoms and subatomic particles has led to innumerable inventions.
Why is the Study of Atomic Structure Important?
Primarily, the atomic structure of any element can be studied by its constituents. A typical atom is composed of the nucleus – made of neutrons and protons at the centre – and electrons revolving around it.
The total number of protons inside the nucleus and electrons outside is always equal, and it is known as the atomic number. Different elements have different atomic numbers, which lends them their unique characteristics. However, to attain stability, an atom gains or loses energy, resulting in the formation of charged power entities called ions.
What were the Different Models of Atoms?
Many scientists came up with their ideas on the structure of atoms during the 18th and 19th centuries. The views of many scientists on the atomic structure possessed both merits and demerits. However, these led to the emergence of the modern atomic model. The most noteworthy models are as follows:
- Atomic model by John Dalton
- Thomson Model of atom
- Rutherford’s model of atom
- Bohr model of atom
State the Postulates of Dalton’s Atomic Model.
John Dalton, an English chemist, explained that an atom is an energy that cannot be created or destroyed but can be transformed from one form to another.
Postulates of his work
- An atom can never be broken or destroyed
- All entities in the universe are made up of particles called atoms
- Every element has a distinct kind of atom
- The mass of an atom is constant and varies from one element to another
- Atoms of elements actively participate and engage in chemical reactions
What were the Conclusions of the Cathode Ray Experiment?
Sir Joseph John, an English chemist, performed the cathode ray experiment and received the Nobel Prize for discovering “electrons”.
From his experiment, he concluded the following:
- Negatively charged particles are entrenched in positive ones
- Atoms are electrically unbiased and neutral, i.e., positive protons and negative electrons are of the same magnitude
List out Rutherford’s interpretation of the Alpha Ray Scattering Experiment in the Context of the Structure of An Atom.
Rutherford, a student of J. J. Thomson, conducted the alpha ray scattering experiment and discovered the “nucleus”. His experiment concluded that the atom consists mainly of empty spaces
- 1/1000th ray reflected at 180° because of a strong charge at the centre of the atom – nucleus
- The nucleus decides the mass of an atom
- Atoms are spherical
What do you mean by the Electronic Configuration of an Atom?
The electronic configuration is how the electrons are arranged around the nucleus or how well they are distributed in the atomic orbital. An electronic configuration is a definitive form in which electron shells are placed in sequence. For instance, the electronic configuration of helium is 1s².
Explain the various aspects involved in electronic configurations
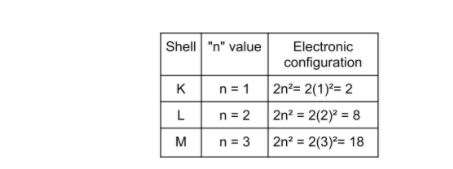
Shells
The electrons that revolve in associate degree orbit are referred to as shells.
- The shell (orbit) is K; the second shell is L
- Therefore, the shells are K, L, M, N, etc
- It is expressed in a formula of 2n², where ‘n’ is the shell number .
Subshells
The subdivision of shells in which electrons are present inside the orbitals is called subshells. It is expressed as ‘l’. ‘l’ is dependent on the value of ‘n’.
- If n = 3, the subshells have three values: l = 0, l = 1, l = 2. Then, the names of the subshells are s, p, d
- If n = 4, the subshells have four values: l = 0, 1, 2, 3. Then, the names of the subshells are s, p, d, f.
- It’s expressed as 2 (2l + l)
What are the Essential Factors in Electronic Configuration of an Atom?
Electronic configuration can be written by labelling the subshells. It contains shell number (n), subshell name (s, p, d, f), and the total number of electrons is written in the form of superscript.
For example, the electronic configuration of helium is written as 1s², and the electronic configuration of elements with atomic number 10 is 1s², 2s², 2p⁶.
List the Principles that are Used While Writing the Electronic Configuration of an Atom
The below three rules show us the proper way of writing electronic configurations.
- Aufbau principle – The orbitals are occupied by the electrons in increasing order. The electrons first occupy the lower energy orbitals before reaching the higher energy orbitals
- Pauli exclusion principle – Two electrons belonging to the same orbital of an element always have opposite spin
- Hund’s rule – One electron must occupy the orbital before the second electron is filled in the same orbital
What is the Significance and Importance of Electronic Configuration?
- The electronic configuration of the element specifies the behaviour of a component
- Properties of elements are studied with the help of their electronic configurations
- The electronic configuration determines the chemical, physical, electrical, and magnetic behaviour of an element
- It determines the chemical reaction in which the elements can participate
- Elements with complete electronic configuration do not react with other elements
- It aids in atomic range arrangement
- It assists in classifying elements in the periodic table
The primary importance and purpose of electronic configuration can be better understood with the help of the following points:
- When two atoms come in contact, the outermost shell reacts first
- The electronic configuration of an element within a filled valence shell is the reason for the chemical behaviour of that element
- Electronic configuration helps in predicting the stability of an electron
- Electronic configuration helps understand an element’s chemical behaviour from the basic hydrogen and helium to the very complex human body proteins
Conclusion
Atoms are the basic fundamental building blocks of matter. Electrons, protons, and neutrons are the fundamental particles of atoms. They are the smallest, inseparable, and invincible particles. The spotting of the Schrödinger equation characterises the void between orbitals as energy levels. The discovery of atom electronic configuration has brought about a significant change in technology and chemistry. The atomic structure and electronic composition help us better understand the matter around us. Understanding these elements helps in forming alloys, compounds, and even the invention of parts.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






