A carbocation is an unstable 6 electron positively charged species. The carbocation formation as an intermediate takes place in the course of the reaction. The carbocation intermediate is formed for an infinitesimally short time interval in unimolecular nucleophilic substitution reactions, unimolecular elimination reactions, electrophilic addition reactions of the alkenes and rearrangements involving hydride shift or alkyl shift. Some important named rearrangement reactions also involve the carbocation formation, for example, Wagner Meerwein rearrangements, etc. Other essential chemical reactions such as reactions involving dehydration of alcohols, hydroboration oxidation reaction, oxymercuration demercuration reactions also involve the carbocation formation.
Mechanism for carbocation formation
A carbocation is generated in one of the two common ways.
The first method involves the heterolytic bond cleavage followed by removing a leaving group. First, the ionisation of the bond connecting the carbon atom and the leaving group occurs. After that, the leaving group and the bonding electrons get removed from the molecule.
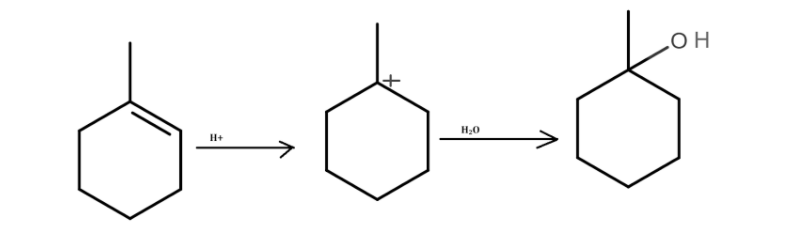
The second method involves the addition of an electrophile (such as H+) to an unsaturated bond (such as an alkene). Due to the addition of this electrophile, the unsaturated bond breaks, and adjacent carbon becomes electron deficient. The breaking of the unsaturated bond leaves the carbon with a positive charge.
It should be noted that the positively charged carbocation has sp2 hybridisation. The carbocation has an empty p-orbital, unutilised and represented with a positive charge. This representation indicates that the empty orbital can accept electrons.
Unimolecular elimination reactions
The most common reaction where carbocation is formed as an intermediate is dehydration of alcohol. Primary alcohol undergoes elimination via the E2 mechanism since primary carbocation is highly unstable. Secondary and tertiary alcohols follow the E1 mechanism to yield alkenes. A proton (H+) source such as sulphuric acid is used to dehydrate alcohols. The carbocation formation occurs when the leaving group is lost from the site.
Dehydration of alcohols: The hydroxyl group (OH) acts as the leaving group, forming a carbocation. The carbocation undergoes a 1,2-hydride shift to form a highly stable tertiary carbocation intermediate. Alkene is formed in the final step.
Addition reactions
There are some common addition reactions of alkenes, such as the addition of alkyl halide, water, or halogen that involve the formation of the carbocation.
Other essential reactions in this category that involve carbocation formation are hydroboration oxidation and oxymercuration demercuration reactions. The carbocation formation takes place via heterolytic cleavage of the unsaturated bond of alkenes
Addition of alkyl halides
Halides are inserted in unsaturated carbon double bonds in the presence of hydrochloric acid. The formation of the carbocation intermediate occurs in the course of the reaction. An alkyl halide is produced as the product of the reaction.
Addition of water
Hydroxyl is inserted in unsaturated carbon double bonds in the presence of water. The formation of the carbocation intermediate occurs in the course of the reaction. Alcohol is produced as the final product of the reaction.
Addition of halogen
Alkene reacts with bromine to give dibromo alkane. The formation of the carbocation intermediate occurs in the course of the reaction. A three-membered transition state is formed in this reaction.
Hydroboration oxidation
Boron (BH3) is inserted in unsaturated bonds of alkene in hydroboration, and oxidation occurs in the presence of H2O2. When hydroboration and oxidation are done simultaneously, such reactions are known as hydroboration-oxidation reactions.
Oxymercuration demercuration
Hydroxyl group (OH) is inserted in alkene in the presence of Hg(OAc)/H2O and NaBH4. Generally, a mixture of two major and minor products is formed because the intermediate carbocation can rearrange itself to form a more stable carbocation.
Unimolecular nucleophilic substitution reactions
The unimolecular nucleophilic substitution reactions or SN1 reactions are slow. The step in which the carbocation intermediate is formed is the rate-determining step of the reaction. The formation of the carbocation facilitates the inversion of the products. The carbocation formation takes place when the leaving group is lost from the site.
The leaving group detaches from the carbon in the given reaction, leaving behind a 6 electron highly reactive carbocation intermediate. Due to the presence of carbocation intermediate, inversion takes place, and the incoming nucleophile is attached on the opposite side.
Rearrangements
Carbocations are highly unstable and reactive. The carbocation stability is lowest for primary carbocations. Secondary carbocations and tertiary carbocations are the most stable. For a carbocation to exist, it must be stable enough. Thus to gain stability, carbocations rearrange to adjacent atoms. The rearrangement either takes place by alkyl shifts or by the hydride shifts. Some named reactions that involve rearrangements of the carbocation are Pinacol reactions, Tiffenean Demjanov reactions etc. These reactions are not discussed in the formation of the carbocation notes.
Conclusion
The carbocation formation happens by following any of the two mechanisms discussed in the formation of the carbocation notes. Carbocation is one of the most important intermediates. It is formed in some major reactions such as nucleophilic substitution reactions, elimination reactions, addition in alkene bonds and rearrangements reactions. Before studying these reactions, one needs to understand the stability of carbocations and the types of carbocation formed in different reactions. Wagner Meerwein rearrangements, Pinacol reactions, and Tiffenean Demjanov reactions also involve carbocation formation. A thorough understanding of carbocation is required to understand these reactions.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out