Introduction
Every second, thousands of chemical interactions take place in our body. Several chemical processes are necessary for life to exist. In order to make new bonds, many molecules have attained stable states and must be ripped apart. However, breaking and establishing bonds takes a lot of energy, which is referred to as ‘activation energy’. The given activation energy is the smallest amount of external energy needed to transform a reactant to a product.
In other words, during chemical reactions, bonds are broken and new bonds are formed. The energy needed to break these bonds and produce products is known as activation energy. A higher value of activation energy implies a reaction which will take place slowly because of higher needs. Whereas a lower value of activation energy means that the reaction will take place easily, since bonds can be easily broken down.
To detail the factors affecting activation energy, we will understand the rate of reaction i.e. the rate at which reactants produce products as a result of a chemical reaction. It gives some information about the time restriction for performing a response. For instance, in a fire, the response rate of cellulose combustion is extraordinarily fast, with the process being completed in less than a second.
Multiple factors affecting activation energy indicate numerous chemical reactions. Hence, many biological processes do not occur spontaneously and require an initial input of energy (activation energy) to begin. When studying both endergonic and exergonic processes, activation energy must be taken into account. Although exergonic reactions create a net energy release, they nonetheless require a small quantity of energy to progress through their energy-releasing phases. Activation energy (or free energy of activation) is the minimum amount of energy that is required for all chemical reactions to occur, and it is denoted by the abbreviation ‘EA’.
Chemical Characteristics of Reacting Substances
The pace of a reaction is determined by the sort of chemicals involved. Reactions that appear to be comparable under the same circumstances may have varying rates depending on the identity of the reactants. When microscopic particles of the metals iron and sodium are exposed to air, the sodium entirely interacts with the air over night, whilst the iron has little effect. Calcium and sodium are both active metals that react with water to form hydrogen gas and a base. Calcium, on the other hand, responds slowly. But sodium reacts so quickly that the process is almost explosive.
Factors Affecting Activation Energy and Reaction Rate
There are several factors affecting the activation energy of a chemical process which is related to its rate of reaction i.e. how fast or slow the products are created. With a rise in activation energy, the chemical reaction will be slower. This is because molecules can only complete the reaction once they have crossed through the barrier of activation energy. The bigger the barrier, the fewer will be the amount of molecules that can pass it at the same moment.
So how come some molecules are more energetic than others? Certain reactions hold such high activation energies that they do not occur in the absence of energy. For example, while the combustion of a fuel, such as propane, generates energy, the rate of reaction is nearly nil at room temperature. (However, this is a good thing – picture if propane canisters exploded on the shelf!) When a spark produces enough energy to drive certain molecules beyond the activation energy barrier, the reaction is completed and energy is released. This released energy helps other fuel molecules break past the energy barrier, resulting in a chain reaction.
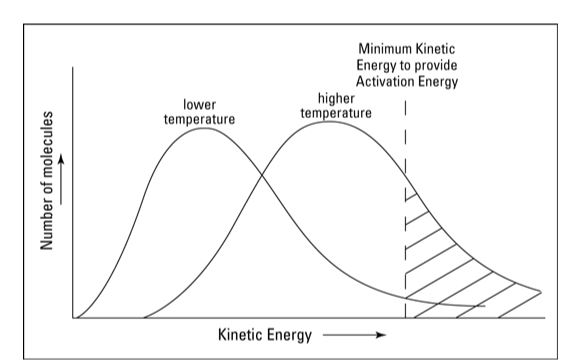
Temperature
Temperature rises often enhance the pace of response. The average kinetic energy of the reactant molecules increases as the temperature rises. As a result, a larger fraction of molecules will have the minimal energy required for an effective collision (see ‘Temperature and Reaction Rate’).
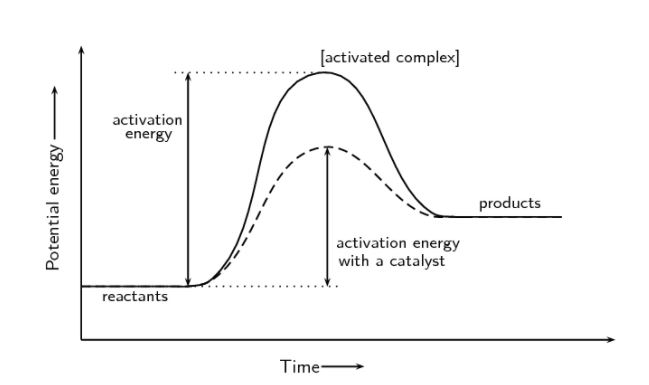
Presence of a Catalyst
A catalyst is a chemical that participates in a process without being consumed. Catalysts give a second reaction route for obtaining products. They play an important role in many biological events. They will be looked at in further detail under the section ‘Catalysis’.
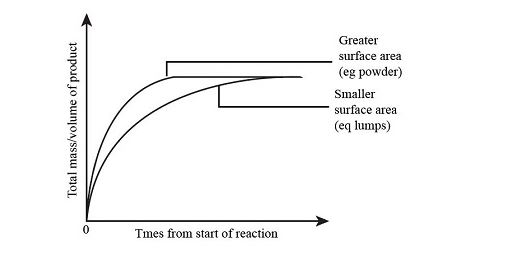
Physical Condition and Surface Area of the Reactants
The surface area of the phases in contact limits the pace of reaction when reactant molecules reside in different phases, as in a heterogeneous mixture. Only the molecules on the metal’s surface may collide with the gas molecules when a solid metal reactant and a gas reactant are mixed. As a result, increasing the surface area of the metal by pounding it flat or slicing it into multiple pieces can improve its reaction rate.
Activation Energy Formula
The given activation energy is compatible and equal to the difference between the threshold energy of the reaction and estimated kinetic energy of all reacting molecules in reactant species.

Hence, if the activation energy of a reaction decreases, the effective collisions’ proportions will be big, have a high amount of energy. Moreover, the pace of the reaction will be high. When the activation energy is large, the number of effective collisions is minimal, and the response rate is sluggish.
So, to summarise:
- Low activation energy results in faster responses.
- Slower reactions due to high activation energy.
- According to the Arrhenius notion, activation energy is affected by a variety of elements, the most significant of which is temperature. The Arrhenius equation is concerned with the quantitative foundation of the link between activation energy and the rate of a chemical process. The formula for activation energy is as follows:

The notations in the above equation are detailed below:
- A serves as the pre-exponential factor;
- T is the absolute temperature (Kelvin), and k denotes the reaction rate coefficient;
- Ris is the Universal Gas constant, while Ea is the activation energy;
The Arrhenius equation is widely recognised as the most accurate experimentally obtained factor for indicating the responsiveness of the reaction rate to temperature and activation energy.
Activation energy is the capacity barrier or energy barrier that reactant molecules must overcome in order to continue in a chemical reaction.
Conclusion
There have been reported approaches for directly determining the activation energy of a chemical system at nearly any dynamical time scale from simulations at a single temperature. In contrast to the standard Arrhenius analysis, which computes the derivative numerically, these methodologies compute the analytical derivative with respect to temperature immediately. They are essentially a dynamical application of statistical mechanics’ fluctuation theory.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



