Introduction:-
When we talk about a balanced chemical reaction, we mean that each element on both sides of the equation has the same number of atoms. The concept of equilibrium concentration is based on these balanced chemical reactions. When the reactants and products of a chemical do not depend upon time, we say it is at equilibrium concentration. Chemical equilibrium, also known as equilibrium concentration, occurs when the rate of forward reaction in a chemical process equals the rate of backward reaction. At the same time, the products and reactants remain unchanged, indicating that the reaction has halted.
Equilibrium Constant:-
To understand how to use the equilibrium concentration equation to compute equilibrium concentration, you must first grasp the formula of equilibrium constant Kc . The equilibrium constant is the ratio of products to reactants when the chemical is in equilibrium. Consider the following chemical reaction:
aA + bB ———–› cC + dD.
The equilibrium constant(Kc) for the above given equation as follow:

Calculation of equilibrium concentration :-
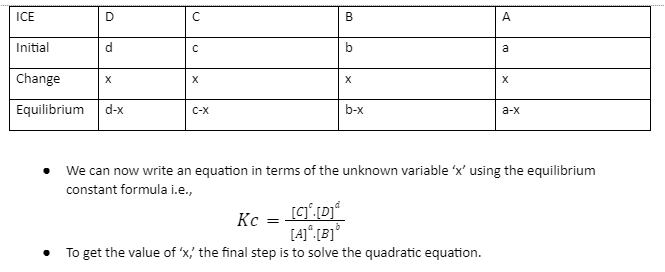
Using the ICE table to find the equilibrium concentration equation is the easiest method. It’s a well-organized table that shows how much of each product and reactant is given and how much needs to be found. When we look at how to compute equilibrium concentration, the equilibrium constant and table will come in handy. Initial concentration is denoted by the letter ‘I.’ The letter ‘C’ stands for concentration change. Equilibrium concentration is denoted by the letter ‘E.’
To determine the equilibrium concentration of a chemical process, a few steps must be followed. The procedure is as follows.
- The first step is to write down the chemical reaction’s balanced equation as
aA + bB ———–› cC + dD.
- The second step is to convert the concentrations of the products and reactants into Molarity units.
- Forming the ICE table and determining what quantities are given and what has to be found is the third stage.
Le-Chatelier’s Principle:-
The Equilibrium Law, commonly known as Le Chatelier’s principle, is a principle in chemistry that predicts the influence of a change in conditions on chemical equilibrium. The principle is named after French scientist Henry Louis Le Chatelier, however Karl Ferdinand Braun is frequently credited with discovering it independently. It can be expressed as follows:
When a system that has been in equilibrium for a long time is subjected to a change in concentration, temperature, volume, or pressure, it
(1) shifts to a new equilibrium, and
(2) this shift partially counteracts the applied change.
It’s customary to think of the idea in terms of a broader observation of systems, such as
- When a well-established system is disrupted, it will adjust to lessen the impact of the change.
The concept of systemic maintenance of an equilibrium state despite perturbations is known by a variety of names, depending on the discipline (for example, in biology, homeostasis is a term that encompasses the concept) and has been studied in a variety of contexts, primarily in the natural sciences. This principle is used in chemistry to alter the responses of reversible processes, usually to increase their yield. According to Le Chatelier’s principle, the binding of ligands to receptors can modify the equilibrium in pharmacology, explaining the various processes of receptor activation and desensitization. The notion has been generalized in economics to aid in the explanation of price equilibrium in efficient economic systems.
In systems of this type, phenomena that appear to defy Le Chatelier’s principle might occur.
Effect of change in pressure:-
The liquid and solid phases are unaffected by pressure variations. Pressure, on the other hand, has a significant impact on the gas phase. According to Le Chatelier’s principle, increasing the pressure pushes the equilibrium towards the side of the reaction with lesser moles of gas, while decreasing the pressures pushes the equilibrium towards the side of the reaction with more moles of gas. Pressure has no influence if the amount of moles of gas on both sides of the reaction is the same.
Effect of change in temperature:-
The type of reaction determines whether it is exothermic or endothermic. Remember that endothermic means that a chemical process absorbs energy, whereas exothermic means that the reaction releases energy. As a result, energy can be conceived of as either a reactant or a product of a reaction:
Endothermic: energy + reactant ————-› product
Exothermic: reactant ————-› product + energy
Increased temperature can be thought of as adding energy because temperature is one of the measure of the system’s energy. The reaction will act as though a reactant or a product is being introduced, shifting to the opposite side as a result. When the temperature of an endothermic reaction is raised, it effectively adds a reactant, shifting the balance toward products. When you lower the temperature, you’re lowering a reactant (in endothermic processes) or a product (in exothermic reactions), and the equilibrium adjusts correspondingly.
Conclusion:-
The principle of Le Chatelier can be used to forecast how a stress, such as changing concentration, will affect an equilibrium response system. When the concentration of a reaction species is raised (at constant V and T), the equilibrium system shifts in the opposite direction, lowering the concentration of that species.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





