The reaction between a strong/weak acid and another strong/weak base to form salt and water is called a neutralisation reaction.
Acid + Base → Salt + Water
For example,
HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
The reaction liberates heat to form H2O in the liquid state. Hence, it is an exothermic reaction. Enthalpy associated with any reaction is called reaction enthalpy. Thus, the enthalpy of neutralisation is the heat liberated when acids react with bases to form salt and water. The SI unit is kJ.
Since water (H2O) is the constant byproduct in any given acid-base reaction, the heat of neutralisation is determined against every mole of water. The heat liberated for the production of every mole of water in a neutralisation reaction is called the Molar Enthalpy of Neutralisation. SI unit is kJ mol-1
Measuring enthalpy of neutralisation
The heat of neutralisation can be experimentally measured using a calorimeter.
Measurements of enthalpy are conducted in two different conditions:
- At constant volume (in this case, the enthalpy of reaction is equal to change in internal energy)
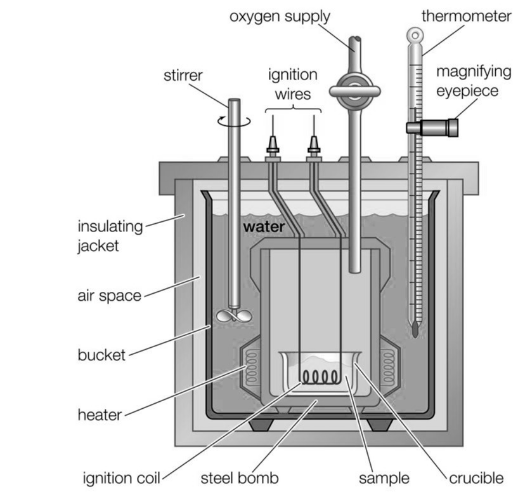
Reactants are placed inside the inner vessel, and when the neutralisation takes place, the heat liberated is absorbed by the surrounding water. Therefore, by calculating the heat gained by the water from its temperature rise, we can know the enthalpy of neutralisation.
The heat capacities of the calorimeter vessel and water are known. The total heat capacity of the system is
C = C cal + C w.
The temperature rise for the calorimeter and water will be the same, ΔT.
Heat gained by the calorimeter will be the product of total heat capacity and temperature differential.
Q = C. ΔT
Q = (C cal + C w) ΔT
Enthalpy of neutralisation = – Q
- At constant pressure
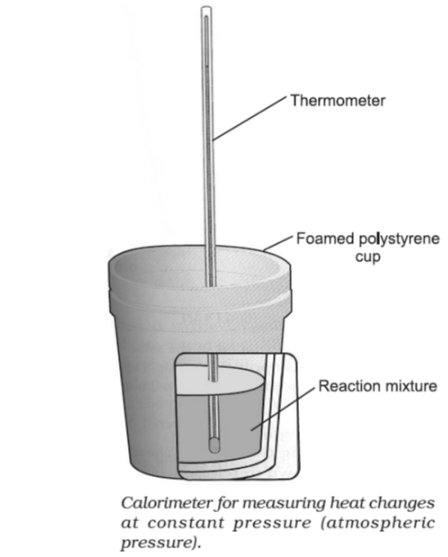
When neutralisation is carried out at atmospheric pressure, the thermometer measures the temperature rise, as depicted in the image.
In this case, enthalpy change can be calculated as:
ΔH = ∑ enthalpies of products – ∑ enthalpies of reactants
Factors affecting enthalpy of neutralisation
The value of enthalpy of neutralisation depends on the strength of the reactant acids and bases in a particular reaction.
If the reactants are strong acids/bases, complete dissociation of molecules takes place. But in the case of a weak acid or weak base, some of the liberated heat is utilised to break the molecular bonds. Hence, the absolute value of enthalpy decreases with the strength of acids and bases. This can be depicted in the table below.
The heat of neutralisation between different acids and alkalis
Acid | Alkali | Heat of neutralisation |
HNO3 | NaOH | 57.3 |
1/2H2SO4 | NaOH | 57.3 |
HCI | KOH | 57.3 |
HNO3 | KOH | 57.3 |
HNO3 | 1/2Ca(OH)2 | 57.3 |
H2S | NaOH | 16.0 |
HCN | NaOH | 12.0 |
HCI | NH3 | 51.5 |
CH3COOH | NaOH | 55.0 |
The dissociation of acids and bases can be understood using the Arrhenius, Brönsted-Lowry, and Lewis theory of Acids and Bases.
Arrhenius stated that acids are substances that dissociate in water to produce hydrogen ions (H+), and bases are those substances that dissociate to produce hydroxyl ions (OH-). However, this theory is limited to aqueous solutions and does not consider the basicity of substances, like ammonia NH3, that do not possess a hydroxyl group.
However, this theory is helpful in understanding the dissociation of ionic compounds whose ionisation mostly occurs in aqueous mediums. For example, acids like Hydrochloric acid (HCl), Perchloric acid (HClO4), Hydrobromic acid (HBr), Nitric acid (HNO3), and Sulphuric acid (H2SO4) are termed strong acids because they fully dissociate into their constituent ions in an aqueous medium.
Similarly, strong bases like Calcium Hydroxide (Ca (OH)2), Lithium Hydroxide (LiOH), Potassium Hydroxide (KOH) and Sodium Hydroxide (NaOH) fully dissociate in an aqueous solution to give hydroxyl ions.
The Brönsted-Lowry theory gave a more comprehensive definition of acids and bases, stating acids are substances that donate hydrogen or H+ ions and bases are the substances that accept hydrogen or H+ ions. In other words, acids are proton donors, and bases are proton acceptors. The pairs of acid-bases that differ by only one proton are together termed as conjugate acid-base pairs. If the Brönsted acid is strong, its conjugate base will be weak and vice-versa.
On the one hand, strong acids like HClO4, HCl, HNO3, and HBr have weak bases like ClO4-, Cl-, NO3-, and Br-; on the other hand, nitrous acid, HNO2, Hydrofluoric acid, HF, and acetic acid, CH3COOH, are weak acids, and they have strong conjugate bases- NO2-, F- and H- that are very good proton acceptors.
The Lewis theory defines an acid as the electron-accepting species and a base as that which donates an electron pair, i.e. lone pair. E.g. if BF3 reacts with NH3, BF3 acts as acid even though it does not have a proton to donate.
BF3 +:NH3 → BF3:NH3
Electron-deficient species like AlCl3, CO3+, Mg2+, etc. can act as Lewis acids while species like H2O, NH3, and OH- etc., which can donate electron pairs, act as Lewis bases.
Factors affecting acid strength
The extent of acid dissociation mainly depends on strength and polarity. When the strength of the HA bond decreases, the energy required to break the bond also decreases, and consequently, HA becomes a stronger acid. When HA becomes more polar, i.e. the electronegativity difference between the atoms H and A increases, there is clear charge separation, and cleavage of this bond becomes easier.
As the size of the elements increases, the acid strength also increases.
Size increases HF << HCl << HBr << HI, acid strength increases
Electronegativity of A increases: CH4 < NH3 < H2O < HF, acid strength increases
Conclusion
Enthalpy of neutralisation depends on the degree of dissociation of acids and bases in a reaction mixture, which in turn depends on their strength.
The Arrhenius theory explains the dissociation of ionic compounds, while the Brönsted-Lowry theory explains the dissociation of weak acids and bases. The degree of dissociation of an acid depends on its strength and polarity. Acidity increases with the increase in size and electronegativity of the atoms.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





