Diazonium salts are organic compounds containing three nitrogen atoms and an alkyl or an aryl (benzene ring) group on the opposite side. The diazonium salts are between the azo dyes and the dye salts (colouring agents). They are referred to as salts due to the presence of a double nitrogen atom (diazo), which is often observed in ionic salts when chloride molecules take the nitrogen atom’s place. The entire procedure for producing diazonium salts is relatively simple.
Definition of diazonium salts
Diazonium salts are referred to as diazonium compounds. Diazonium salts are organic molecules with the formula R–N2+X–, where R is an alkyl or aryl group, X is any halogen or organic compound. Aryl diazonium salts are frequently used as intermediates to synthesise organic compounds.
Arenediazonium salts are a form of the chemical arene diazonium. In the term ‘Diazonium salts,’ ‘di’ denotes two, aza represents nitrogen, and onium represents the compound’s ionic nature. As a result, ionic substances containing N≡N are referred to as diazonium salts.
Properties of diazonium salts
- They are naturally ionic.
- Diazonium salts are colourless crystalline compounds. If they are exposed to the air, they darken.
- Many nitrate and perchlorate diazonium compounds explode when overheated or heat dried. Since they are synthesised in situ, these compounds are not segregated and can be immediately added to various synthetic preparations.
- Double salts of diazonium and zinc chloride, along with diazonium and trifluoroborates, are stable at room temperature and have been used as faster dye salts in naphthol synthesis.
The significance of diazonium salts
- Diazonium salts are commonly used chemicals in synthetic organic chemistry. Earlier, diazonium salts were used to create water-fast coloured fabrics, and the reaction comprises immersing textiles into water-solvent solutions.
- Then it was immersed in a coupler solution, an electron-rich ring that performs electrophilic substitution processes.
- Diazonium salts are typically adopted in the pigment and dye industries to create dyed fabrics.
- Presently, diazonium salts are frequently used to synthesise azo dyes, and as a result, they are critical in industry and organic chemistry.
- Diazonium compounds, especially aryl derivatives, are widely used in chemical synthesis. Benzene cannot be directly substituted for aromatic compounds; hence diazo compounds are substituted for these compounds in diazonium salts.
- Azo pigments are similarly synthesised from diazonium salts and share a similar chemical structure as azo dyes. These azo pigments play a significant role in various sectors, including paints, plastics, and rubber materials. They are popular because of their light-fastness and the high quality of their colouring pigments.
The methodology for preparing diazonium salts
The diazotisation or dissociation of an organic compound, most commonly primary aromatic amines, results in the formation of diazonium salts. Diazonium groups are extremely unstable and so cannot be stored. As a result, we usually use them instantly after they’ve been prepared. Nitrous acid reacts with aromatic amines to form the most stable diazonium salt.

Aniline (aromatic amine) and nitrous acid produce the diazonium salt. Nitrous acid is a highly hazardous gas. NaNO2 and a mineral acid are typically prepared throughout the reaction in which they are used.
Reactions of diazonium salts
This is vital since the produced diazonium salts can be converted into an infinite lot of excellent functional groups.
The term “versatile” can be applied to any process that uses a single raw material and can be converted into seven different end products.
The diazonium salt reactions can be broadly classified into two types: Sandmeyer’s reactions, among many other responses.
1. Sandmeyer Reactions:
One method of transforming diazonium salts is to treat them with different copper compounds. Sandmeyer’s reactions are named after Traugott Sandmeyer, who discovered them in 1884.
Three significant examples include the following:
- CuCl converts aryl diazonium salts into aryl chlorides
- CuBr converts aryl diazonium salts into aryl bromides
- CuCN converts aryl diazonium salts into aryl cyanides.
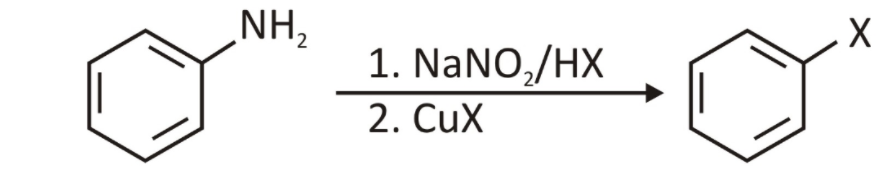
According to this reaction, Aryl radicals are oxidised to aryl cations, which are subsequently attacked by nucleophiles.
2. Other Reactions:
Substitution occurs without copper if a strong nucleophile is present and the mixture is heated sufficiently:
- Aryl iodides can also be synthesised by treating aryl diazonium salts with potassium iodide (KI).
C6H5N2Cl + KI →C6H5I + KCl + N2
- By heating an aryl diazonium salt with water and acid, hydroxyl (OH) groups can be added.
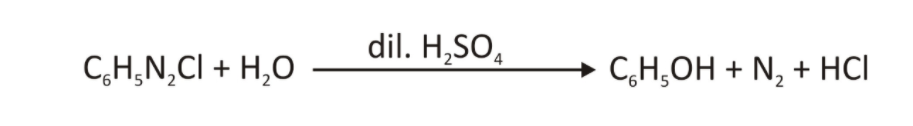
- Aryl fluorides are installed in two steps. By processing the most stable diazonium salt with HBF4, the counter (X–) ion on the aryl diazonium salt is exchanged for the tetrafluoroborate (BF4–) ion. Fluorine can then serve as a nucleophile, replacing N2 and generating BF3 when heated.
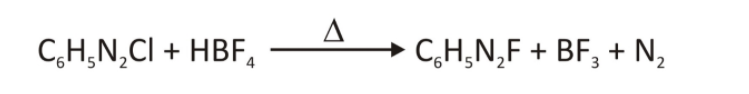
- The aryl diazonium salt can be converted to C–H by treating it with hypophosphorous acids (H3PO2).
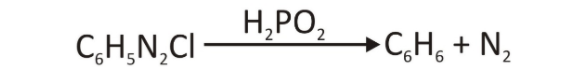
- Diazo coupling processes make synthetic dyes like yellow, red, and orange. In this context, they are referred to as azo dyes due to their ability to give colour and exist in both -cis and -trans formations.
C6H5N2Cl + C6H5OH→C6H5 –N2–C6H4 –OH
Conclusion
Diazotization is the chemical process of converting a primary aromatic amino group into its diazonium salt. Typically, these diazonium salts are prepared by reacting an aromatic amine, including nitrous acid, with another compound. However, it has been discovered that the diazonium group is extremely unstable under normal settings. As a result, it is not usually stored; instead, it is used immediately after preparation. Therefore, nitrous acid and aromatic amines are commonly used to synthesise diazonium salts.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




