Practical functional groups are assortments of iotas in natural science atoms that add to the synthetic qualities of the particle and take part in unsurprising responses. These functional groups of molecules contain oxygen or nitrogen or in some cases, sulfur joined to a hydrocarbon skeleton. Natural physicists can educate a ton concerning a particle by the practical functional groups that make up an atom.
This short rundown contains a significant number of the most widely recognized natural practical functional groups.
1. Hydroxyl Functional Group:
An O-H group that consists of single hydrogen covalently bonded with oxygen is known as hydroxyl. Alcohols and carboxylic acids are organic compounds that contain one or more hydroxyl groups.
2. Aldehyde Functional Group:
A compound that has the -CHO group attached is known as the aldehyde. It consists of a carbonyl centre with the carbon atom also bonded to hydrogen and to any generic alkyl or side chain R group.Aldehydes have the formula R-CHO.
3. Ketone Functional Group:
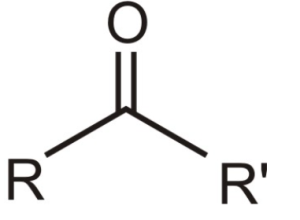
When the carbon of a carbonyl group is bonded to two other carbons such compounds are known as ketones. Ketones are named the same way as are alkenes except that an -one end is used. We can locate the carbonyl group in the molecule by numbering the longest chain of carbons so that the carbonyl group has the lowest number possible.
4. Amine Functional Group:
When NH3, a derivative of ammonia, is added as a functional group, such a compound is known as amine.
5. Amino Functional Group:
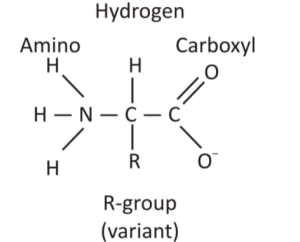
The amino group is a compound that has a nitrogen atom attached by single bonds to hydrogen atoms. Amines are compounds that have an amino group attached. Similar to oxygen, nitrogen is also more electronegative than both carbon and hydrogen which is the reason behind polarity.
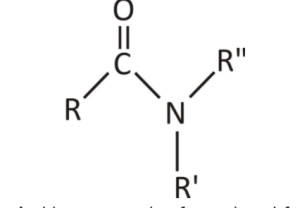
6. Amide Functional Group:
Amides are a mix of a carbonyl functional group and an amine functional group.
7. Ether Functional Group:
An ether functional group comprises an oxygen particle that forms single bonds with two carbon atoms. Ethers have equation ROR.
8. Ester Functional Group:
The ester functional group is a carbon bound to three other atoms. Esters have the equation RCO2R.
9. Carboxylic Acid Functional Group:
Otherwise called the carboxyl functional group. The carboxyl functional group is an ester where one substituent R is a hydrogen particle.
The carboxyl functional group is typically signified by – COOH
10. Thiol Functional Group:
A compound that contains the –SH functional group, which is the sulfur analogue of a hydroxyl or alcohol group is a thiol. It is either known as thiol or sulfhydryl group. They are also called Mercaptans.
11. Phenyl Functional Group:
A cyclic group of atoms with the formula C6H5 i is known as the phenyl group. Benzene and Phenyl are closely related and can be seen as a benzene ring, minus hydrogen, which may be replaced by some other element or compound to serve as a functional group.
Test for Alcoholic functional group (R-OH)
a. Ceric ammonium nitrates- Yellow to Reddish Brown
For Phenolic Group(AR-OH)
a. Ferric chloride test:
In a test tube, we have added 2 mL of an organic compound which is an aqueous/ alcoholic solution. Later, we have to add a dropwise ferric chloride solution which is neutral in nature. As the blue, green, violet or red colour appears it shows the presence of phenolic or OH group
b. Phthalein colour test:
Add 0.1 g of organic compound along with 0.1 g of phthalic anhydride in a clean dry test tube and add 1-2 drops of conc. H2SO4. In an oil bath, heat the test tube for about 1 minute. Let the mixture cool and pour it carefully into a beaker that has 15 mL of dilute sodium hydroxide solution. The pink, blue, green, red, etc appear. The colours are the indication of the presence of phenolic –OH groups in the compound. However, the colour disappears in addition to a large excess of sodium hydroxide solution.
Aldehyde and ketonic Groups(- CHO, – CO-H)
a. 2,4-Dinitrophenylhydrazine test (2,4-DNP test):
Take 2-3 drops of the liquid compound in a test tube or in the case of a solid compound, dissolve a few crystals of it in 2-3 mL alcohol. An alcoholic solution known as 2,4-dinitrophenylhydrazine needs to be added. Yellow, orange or orange-red coloured precipitate confirms the presence of the carbonyl group. Warm the mixture in a water bath and let it cool if precipitate does not appear at room temperature
Note: This test is given by both Aldehydes and Ketones.
b. Schiff’s test:
Add the 2 to 3 droplets of Schiff’s reagent after taking a liquid compound’s 3-4 drops or dissolve some crystals in alcohol.
The mixture shows pink colour which confirms the presence of an aldehyde.
c. Fehling’s test:
In a clean dry test tube take almost 1 mL of Fehling solution A and Fehling solution B. Now, add either the liquid organic compound or the solid compound after dissolving it into water or alcohol. As the brick red precipitate appears it shows the formation of copper (I) oxide and hence aldehyde is present.
The aromatic aldehyde does not undergo Fehling’s test.
d. Tollen’s test:
(i) Prepare silver nitrate solution and take 1 mL of it (~2%) in a test tube. We have to add 1 or 2 drops of sodium hydroxide in the test tube and shake well, it forms a precipitate of dark brown colour made by silver oxide. Now add the drops of ammonium hydroxide solution to the precipitate.
(ii) Now in the above solution, add the organic solution dissolved either in water or alcohol.
(iii) The mixture of steps
(iv) Needs to be heated with the help of a water bath for 5 minutes approx. Silver metal forms a layer on the inner surface and shines like a mirror in a test tube, this confirms the presence of aldehydes.
5. Carboxyl Group(- COOH)
a. Sodium hydrogen carbonate test:
By using a glass rod, put a drop of the liquid compound or a drop of the solution of the compound on a moist blue litmus paper.
As the colour transition occurs from blue to red it confirms the presence of either a carboxylic group or a phenolic group.
b. Ester test:
In a test tube, adding 0.1 g of the compound needs to be tested. With concentrated sulphuric acid add either 1 mL of ethanol or methanol. The mixture needs to be heated indirectly with the help of a water bath at a temperature of about 50° C. Take a beaker that has aqueous sodium carbonate solution and pour the reaction mixture into it which neutralises the excess sulphuric and carboxylic acid. The product formed has a sweet smell and confirms the presence of a carboxyl functional group.
6. Amino Group(- NH2)
a. Carbylamine test:
The carbylamine test is given by both aliphatic as well as aromatic amines. In this test, the amine is heated with chloroform
R-NH2 + CHCl3 + 3KOH →∆ RNC + 3KCl + 3H2O (R=alkyl or aryl group) (Carbylamine)
Conclusion:
- To determine what functional group is present in the given compound you have to first check whether the compound is saturated or unsaturated, for we have baeyer’s test and bromine water test.
To test for the Alcoholic group, we have the Ferric ammonium nitrate test, iodoform test, Lucas test.
- To test for the Phenolic group, we have the Ferric chloride test and the Phthalein colour test.
- To test for Aldehyde and ketone groups, we have 2,4-DNP TEST, Schiff’s test, Fehling’s test and Tollen’s test.
- To test for the Carboxyl group, we have a sodium nitrogen carbonate test and an Ester test.
- To test for the Amino group, we have a dissolvability test and a carbylamine test.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





