Introduction
Detecting the elements contained in organic compounds is an important step in their analysis. All organic compounds contain carbon. Hydrogen is also found in most organic compounds (with a few exceptions, compounds such as CCl4 and CS2). In addition to carbon and hydrogen, oxygen, nitrogen, sulfur and halogens are other elements commonly found in organic compounds. Almost all organic compounds contain carbon in addition to hydrogen, so it is usually not necessary to run tests to detect them, and their presence can be suspected without testing. Only tests to detect nitrogen, sulfur, and halogens are examined here.
Carbon is the main component of all organic compounds. Hydrogen is also contained in most organic compounds, with some exceptions such as CCl4 and CS2. Elements other than these elements, such as nitrogen, sulfur, and halogens (chlorine, bromine, iodine), are also contained organically. Compound. These additional elements are usually detected by the Lassaigne test conducted by the French chemist J.L. Lassaigne. In this test, organic compounds are fused with metallic sodium to convert these elements into water-soluble sodium salts. Regular qualitative tests are performed on this extract to find the corresponding element.
Body
Preparation Of Lassaigne’s Extract
Place a small piece of dry sodium in a fusion tube. Gently heat the tube until it melts into shiny beads. Add a pinch of organic compound. First, heat the compound slowly so that it reacts with the sodium metal. Now heat it strongly. Immerse a glowing tube in a porcelain bowl of distilled water. Crush the contents with a glass rod and bring to a boil. Insoluble substances are removed by filtration. The filtrate is called Lassaigne extract. Nitrogen, sulfur and halogens present in organic compounds are detected with the help of Lassaigne extract.
Procedure
Put 15 cm3 of distilled water in a clean porcelain bowl and place it near your burner. Add about 20 mg of sample to the bottom of a small fusion tube or For liquids, place one or two drops of liquid in a fusion tube. Pipette or dropper. Use pliers to hold the fusion tube. Roughly give sodium. Place a 4mm cube in the mouth of the test tube without getting inside the test tube. Contact with substances on the ground. Gently heat the sodium into a small one. Raise the flame until the burner melts and runs down the sample. It can be a very violent reaction when molten sodium comes into contact with the sample. Heat the pipe gently for 1 minute, heat until the bottom of the tube glows. It glows red. Hold the cheesecloth freehand with pliers and drop the sparkling fusion. Place the tube in water in a porcelain dish and immediately cover it with a cheesecloth. If the weld hose does not break when exposed to water, crush it with the help of a glass rod. Reacting excess sodium, the reaction when calm, place a porcelain dish on a tripod’s lawn cloth and bring the solution to a boil for 2 minutes. Filter the solution while hot to remove broken glass and char using this aqueous solution as a material, divide it into 5 equal parts in 5 test tubes.
Detection Of Nitrogen
Add 2 ml of freshly prepared iron sulphate solution to a small amount of Lassaigne extract (usually alkaline) and heat. Next, add 2 or 3 drops of FeCl3 solution and acidify with HCl. The Prussian blue color indicates that the compound contains nitrogen.
If nitrogen is present in the compound, the sodium extract is going to contain sodium cyanide formed during melting. When the required reagents are added, sodium cyanide reacts to form Prussian blue iron (III) ferrocyanide.
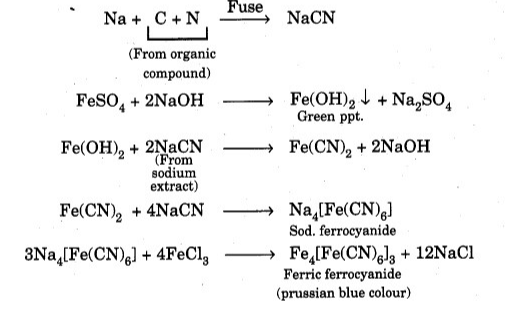
Finally, the purpose of acidifying the reaction mixture is to dissolve the green ppt. Of Fe(OH)2, as it can lead to wrong conclusions. Nitrogen and sulfur coexist. When the organic compound contains both nitrogen and sulfur, the production of Lassaigne extract produces sodium thiocyanate (NaCNS). Sodium sulphocyanide reacts with iron (III) chloride and turns red like blood due to the formation of iron (III) sulphocyanide.
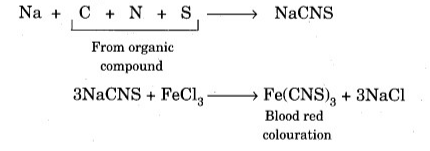
Therefore, the appearance of a blood-red color when running the nitrogen Lassaigne test indicates the presence of both nitrogen and sulfur in the organic compound.
Detection Of Sulfur
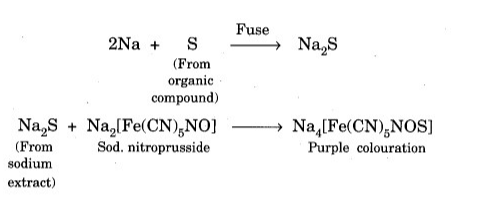
- Sodium nitroprusside test. Add a few drops of C5H4FeN6Na2O3 to a small amount of Lassaigne extract. Purple indicates that the compound contains sulfur.
Lead acetate test:
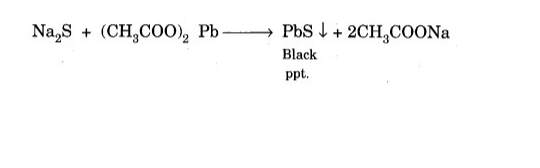
Acidify a small amount of lacene extract with acetic acid and add a few drops of lead acetate solution. Formation of black ppt. Indicates that the compound contains sulfur.
Detection Of phosphorus
Heat the organic compound with an oxidizer. Phosphorus contained in the compound is oxidized to phosphate. The solution is boiled in nitric acid and then treated with ammonium molybdate. Yellow indicates the presence of phosphorus.
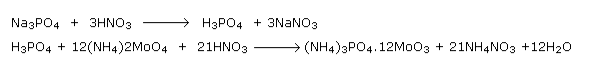
Detection Of halogens
Halogen in organic compounds is detected by the Carius method. Masses of organic compounds are heated with fuming nitric acid in the presence of silver nitrate in the Carius tube. Carbon and hydrogen are oxidized to carbon dioxide and water. The halogens present form silver halides and are finally weighed.
SFE sodium fusion extract can be used to detect the presence of chlorine, bromine and iodine, but not fluorine. To prove their presence, SFE is first acidified with HNO3 and then mixed with AgNO3 solution.
i) The formation of a massive white precipitate soluble in NH4OH indicates the presence of chlorine in the organic compound.
Lassaigne test for chlorine
ii) The presence of bromine is confirmed by the formation of a pale yellow precipitate that partially dissolves in NH4
Lassaigne test of pale yellow bromine precipitate
iii) The formation of a yellow precipitate insoluble in NH4OH confirms the presence of iodine in the organic compound.
Conclusion
Organic compounds contain carbon, usually hydrogen. They can contain one or more elements of oxygen, bromine, iodine, nitrogen, sulfur and phosphorus. Metal may also be present. The proof of these factors is based on the following considerations: Normally, only nitrogen, sulfur, halogens and phosphorus need to be checked. This can be done by running the Lassaigne’s Sodium Fusion Test. Since there are very few organic compounds that do not contain hydrogen, the presence of carbon and hydrogen is possible. The presence of oxygen is usually detected in later functional group tests.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



