Introducion
The detection of numerous elements (Cl, Br, I, S, and N) using a non breakable sodium ignition apparatus (NOSIA) instead of breakable ignition tubes is discussed in this study. The procedure involves heating an organic chemical and sodium metal in the NOSIA’s fusion zone until they melt (100–150°C), then adding water (80–90°C). The resulting mixture was then utilised to directly identify components. When the sodium in the old method was heated, it would frequently leap out of the ignition tube and catch fire, posing a significant concern in the laboratory. NOSIA has been used by many teachers as well as undergraduate, postgraduate, and research students as a safer and resource-saving strategy. The NOSIA method does not require the breaking of glass ignition tubes or the subsequent filtering of the solution. As a result, the NOSIA technique discovers elements and detects functional groupings in a single phase. Because element detection is a common method for compound identification in organic chemistry, efforts have been made to make it a safer and less wasteful process. Volatile, flammable, toxic, or carcinogenic compounds, for example, may escape from a typical breakable ignition tube, but the NOSIA avoids this.
Tests for Different Elements
Test for Halogen
When halogens in an organic substance combine with sodium metal, they create sodium halide. After acidifying with dil. HNO3, sodium halide extracted with water may be easily detected by adding silver nitrate solution.
When chlorine is present, a white curdy precipitate is generated that is soluble in ammonium hydroxide solution.
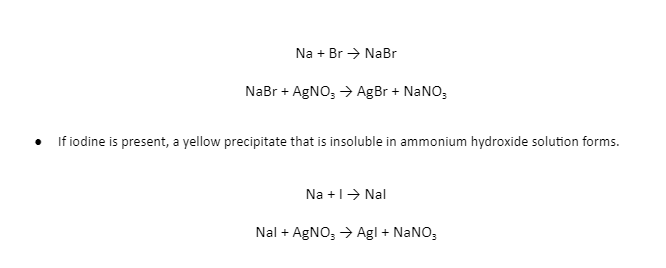
Test for Nitrogen
When the carbon and nitrogen in the organic substance fuse with sodium metal, sodium cyanide (NaCN) is formed, which is soluble in water. The addition of a sufficient amount of ferrous sulphate converts this to sodium ferrocyanide. Ferric ions produced during the procedure react with ferrocyanide to make prussian blue ferric ferrocyanide precipitate.
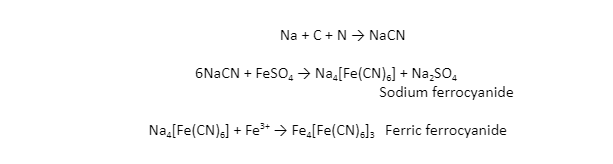
Test for Sulphur
If the organic substance contains sulphur, sodium fusion will convert it to sodium sulphide. Sodium nitroprusside is an excellent tool for identifying sulphide ions.
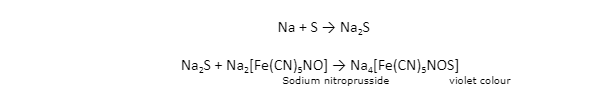
Test for both Nitrogen and Sulphur
If an organic component has both nitrogen and sulphur, sodium fusion will transform it into sodium thiocyanate, which will then combine with Fe3+ to generate the blood colour complex [Fe(SCN)]. 2+

NOTE: If there is an overabundance of sodium metal in the Lassaigne’s extract, sodium cyanide and sulphides are generated instead of sodium thiocyanate.

Procedure
Preparation of Lassaigne’s Extract
In an ignition tube, a little pellet of metallic sodium and a small amount of the material are heated to red hot. It is then submerged in 10ml of distilled water in a china dish. The mixture is thoroughly cooked and strained. The filtrate is used for the tests listed below.
1. Detection of Nitrogen
Add an equal volume of newly made, boiling and cooled FeSO4 solution to a small amount of the filtrate. After that, add a few drops of FeCl3 solution and acidify with dl HCl. The presence of nitrogen is indicated by the appearance of blue precipitate.
2. Detection of Chlorine
Add a few drops of boiling and cooled dil HNO3 to a little amount of the filtrate. Then add the AgNO3 solution. The presence of chlorine is confirmed by the development of a white precipitate that is soluble in ammonium hydroxide.
3. Detection of Sulphur
Add a few drops of newly made sodium nitroprusside solution to a small amount of the filtrate. The presence of sulphur is indicated by the presence of vivid violet colour.
Conclusion
Therefore we can conclude from this whole article that the Experiments for element identification using the old approach are both wasteful and dangerous. The NOSIA methodology is a safer and more cost-effective way, and it is especially useful for volatile liquids, which have proven challenging to handle owing to a variety of issues.
Using NOSIA instead of the standard approach saves 97 percent of the water, power, and other resources. The NOSIA technique was discovered to be a single-step approach for element identification, requiring no extra laboratory infrastructure construction and conserving considerable amounts of consumable resources. The NOSIA might be used to teach green chemistry ideas to students in the 10th and 12th grades, as well as to introduce the importance of additional elements in organic molecules.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




