Introduction
When a non-volatile solute is added to a volatile solvent, the vapour pressure of the solution decreases. The freezing point depression is an example of a colligative property that’s affected by the decrease in vapour pressure. In this article, we will discuss in-depth the freezing point depression, the formula for finding the freezing point depression, molal depression constant, and the real-time applications of freezing point depression.
Colligative Property of a Solution
While studying the properties of the solution, it is crucial to consider the colligative properties. The colligative property of a solution is based on the number of solute particles present in the solution and not on the nature of the solute. The function of the colligative property is to identify the molar mass of the solutes.
The important colligative properties are classified as follows:
- Relative lowering of vapour pressure
- Elevation of boiling point
- Depression of freezing point
- Osmotic pressure
This section will cover the colligative property – depression of freezing point, freezing point depression formula, and the molal depression constant.
The freezing point of a substance
- A substance is said to be at its freezing point if the solid phase and the liquid phase are in dynamic equilibrium with each other.
- In other words, the temperature at which the vapour pressure (the pressure that is exerted by the vapour of a liquid over a liquid phase under equilibrium conditions) of a substance at its solid phase is equal to the vapour pressure of a substance at its liquid phase is defined as the freezing point of the substance.
- Since vapourisation is temperature-dependent, reducing the vapour pressure of a solution decreases the freezing point.
Depression of Freezing Point
- A solution will begin to freeze when the vapour pressures of a pure solid solvent and the solution is equal.
- Freezing Point Depression can be calculated using Raoult’s law. According to Raoult’s law, when you add a non-volatile solid (solute) to a solvent, the vapour pressure of the solvent will decrease. At a lower temperature, the solvent will become equal to that of the solid solvent.
- The formula for depression in freezing point is given by
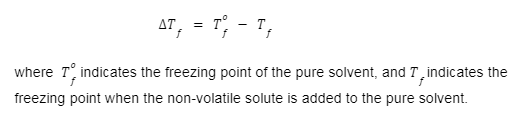
Molal Depression Constant
The depression of freezing point (Tf) of an ideal solution is directly proportional to the molality of the solution. The molality of the solution is denoted by m.
- The Proportionality Constant Kf depending on the nature and type of the solvent is referred to as the Molal Depression Constant. It is also known as freezing point depression constant or cryoscopic constant.
- The unit of Molal Depression Constant (Kf) is K kg mol-1.


Examples of Freezing Point Depression
- In snow-covered roads, the application of salt helps in reducing the freezing point of water, letting the ice melt and clearing the road from snow.
- When preparing ice cream, adding salt to ice lowers the freezing temperature and controls the ice crystal formation. This is due to freezing point depression.
- Freezing point Depression explains why vodka kept in a freezer does not freeze. This is because alcohol has an extremely low freezing point and the presence of alcohol content is comparatively high in vodka.
Conclusion
In this section, we discussed one of the important colligative properties – the freezing point of depression. We also learned that a solution will freeze when the vapour pressure of the solution equals the vapour pressure of the pure solid solvent. Along with the above concepts, we also studied how to calculate the freezing point depression and how to find the molal depression constant.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






