INTRODUCTION:
FACTORS INFLUENCING REACTION RATE:
The study of chemical processes is incomplete unless the speeds at which these reactions occur are considered. We know that certain reactions, such as those involving ions in solution, occur very quickly, while others occur so slowly that the rate is undetectable. The practical significance of these rate issues cannot be overstated. For example, a metal exposed to the elements may corrode as a result of interactions with oxygen and water. Temperature, concentration, and catalysis are three of the most critical elements determining the pace of a reaction. Furthermore, the surface quality of solids is quite important.
In order to explain reaction rates, two major ideas are used. The Activated Complex (Transition State) Theory and the Collision Theory are two of them. However, you will also learn about the Activated Complex (Transition State) Theory throughout the lecture portion of the course.
COLLISION THEORY
Consider the simple reaction: A + B ————-> Products
Because A and B are atoms, ions, or molecules, they must “collide” in order to react with one other. Because molecules are in constant and fast motion, molecules of A and Be will clash at regular intervals.
However, not every A-B collision results in the production of products. Before a reaction to take place, the reactants must collide with a particular level of energy, which is referred to as “activation energy” or “energy of activation.” This energy is derived from the kinetic energy of A and B, hence only collisions with sufficient force will be successful in triggering a reaction. The number of collisions between A and B per time doubles when the concentration of either A or B is doubled. When the temperature rises, the kinetic energy of both A and B increase, resulting in more collisions each second, with a bigger percentage of these resulting in chemical reaction. As a result, the rate normally increases as the temperature rises.
The Arrhenius Equation may be used to calculate activation energy: k = Ae-Ea/RT
The Arrhenius equation is rewritten as ln k = − Ea/RT + ln A.
Where, k is the rate constant
A is the frequency factor.
Ea stands for activation energy.
R = gas constant, expressed as 8.3145 J/mol K
T = absolute temperature, K = Kelvin
When the slope of ln k is plotted against the slope of 1/T, the slope = -Ea/R, and the slope intercept is ln A.
CATALYSIS
A catalyst is a chemical agent that changes the rate of a chemical process. This is due to a reduction in the amount of activation energy required for the reaction. When less activation energy is required, a greater proportion of collisions will have the requisite energy, and the rate will increase. The way the catalyst reduces the activation energy varies on the type of catalyst. An inhibitor is a catalyst that slows down the rate of a process.
CLOCK REACTION
The influence of temperature and concentration on the rate of a chemical reaction will be investigated in this experiment. The selected reaction, sometimes referred to as the “clock reaction,” is really a set of successive reactions represented by the
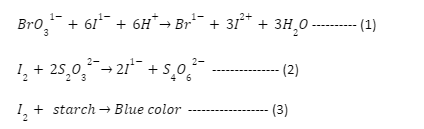
The iodine created in reaction (1) is promptly consumed in reaction (2), therefore no meaningful quantity of iodine may accumulate until all of the Na2S2O3 has been used. When this happens, the iodine content rises to the point where a starch indicator becomes blue. The development of the blue tint indicates that all of the Na2S2O3 has been consumed.
RATE LAW
The rate law of the preceding reaction will be established in this experiment.
Experimentally, the numerical values of x, y, and z will be determined. x, y, and z are also the reaction’s order with regard to A, B, and C. The overall order of the reaction is given by the sum of the individual orders of the reactant. After calculating x, y, and z, the rate constant, k, may be calculated.
Arrhenius Equation
The Arrhenius Equation for the temperature dependence of the rate of a chemical reaction is shown below.
Where,k denotes the reaction’s rate constant.
A stands for Arrhenius. Activation = Constant Ea The reaction’s energy (in Joules mol1)
R stands for Universal Gas. Constant
T denotes absolute temperature (in kelvins)
We knew that when temperature increased, reaction rates increased, but we didn’t know how to anticipate the relationship between temperature and reaction rates statistically. We were able to solve this problem because of the Arrhenius Equation. It is an empirical relationship that is used to simulate the fluctuation of the rate constant with temperature, which offers information about the speeds of chemical processes at various temperatures.
Temperature, concentration, and catalysts influence rates as follows:
Reaction rates tend to increase with temperature: This pattern arises from the fact that reactants must collide with one another in order to react. The reaction can occur if reactants collide with the proper orientation and adequate energy. As a result, the more collisions there are and the greater the energy of those collisions, the more real responding occurs. When the temperature rises, the average kinetic energy of the particles in a reacting mixture rises, causing the particles to travel faster and collide more frequently and with more energy.
Increasing concentration tends to increase the reaction rate: The cause of this tendency is likewise related to collisions. A higher concentration suggests that more reactant particles are closer together, resulting in more collisions and a greater likelihood of reaction. Increasing the concentration of reactants may result in more of those reactants dissolving in solution.
Some reactants are not entirely dissolved and so appear as bigger, undissolved particles. Smaller particles result in speedier responses in certain circumstances. Smaller particles reveal more surface area, exposing a larger fraction of the particle to response.
Catalysts increase reaction rates: Catalysts are not chemically altered, and thus do not affect the quantity of product that a process can eventually create (the yield). An example from childhood is appropriate here. When you’re starting to ride a bike, your parents may give you a push to get started. The pedaling, though, is entirely up to you after that initial push. Your highest speed and final destination are still fully determined by your ability to pedal and control the bike, but that push (the catalyst) aided you in getting up to speed faster.
Catalysts can work in a variety of ways, but all of them involve lowering activation energy, the energetic hill reactants must climb to reach a transition state, the highest-energy stage along a chemical pathway. Lower activation energies result in quicker reflexes.
Conclusion:
Therefore we can conclude from this whole article that when the temperature rises, the average kinetic energy of the particles in a reacting mixture rises, causing the particles to travel faster and collide more frequently and with more energy. Increasing the concentration has the effect of increasing the response rate.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



