One of the most remarkable properties of coordination compounds is the wide range of colours shown by them. It has been a subject of great interest to chemists. Why is it that the hexaaquairon(II) complex appears red, but the hexaaquacobalt(II) complex appears pink and hexaaquanickel(II) complex green? The answer to such questions lies in the crystal field theory of coordination compounds. However, before we learn that, it is crucial to understand why we see colours in the first place. We see the colour in any compound whenever it absorbs visible radiation.
Colour Wheel
You must be aware of the electromagnetic radiation spectrum CGXUVIMR in which radiations are placed in the order of increasing wavelength or decreasing frequency. Whenever light of varying wavelengths is incident on a sample, the sample absorbs radiation of a particular range of wavelengths only. If it absorbs radiation in the visible range, then we see colour complementary to the colour it absorbs.
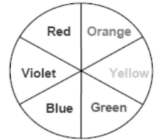
If a sample absorbs blue colour, we see the colour complementary to blue, i.e., orange, and vice versa.
Colour absorbed | Wavelength range (nm) | Colour transmitted |
Red | 700-620 | Green |
Orange | 620-580 | Blue |
Yellow | 580-560 | Violet |
Green | 560-490 | Red |
Blue | 490-430 | Orange |
Violet | 430-380 | Yellow |
If the compound absorbs radiation that does not lie in the visible region, it appears white or colourless.
Colour of Complexes Meaning
The colour of complexes is because of electronic transitions between different energy levels. The transition occurs from one d-orbital to another d-orbital. But how is it possible if all the d-orbitals are equal in energy, i.e., degenerate? d-orbitals of metal are indeed degenerate. However, their energy changes when they come in the vicinity of a ligand.
This can be explained by the crystal field theory (CFT). CFT states that bonding between metal and ligand occurs purely out of electrostatic interactions between the two. As per this, we treat ligands as point charges in the case of anions and point dipoles in the case of neutral molecules. Due to these point charges/dipoles, the degeneracy of the d-orbitals is lost. They split into two different sets of orbitals having different energies. This splitting happens differently in octahedral and tetrahedral complexes.
CFT in Octahedral Complexes
In an octahedral compound, the metal orbital can lie along the x, y, and z axes or in between them. Orbitals dx2-y2 and dz2 point towards the axes along which the ligand approaches and experience more electrostatic repulsion, which causes them to rise higher in energy. On the other hand, the lobes of orbitals dxy, dyz, and dxz lie in between the axes. So, they do not experience repulsion directly from the ligand. As a result, they are lowered in energy.

When the ligand is a strong field ligand, then the energy difference between the two sets of orbitals is higher as compared to when the ligand is weak-field. In the former case, the electrons absorb radiation of a lower wavelength (or higher energy) to transition to the upper energy level. Such compounds appear red, orange, or yellow.
When the energy difference between the two sets of orbitals is low, the electron absorbs low energy radiation, and the compound appears blue, violet, or greenish.
Ligands have been arranged according to their field strength in the below spectrochemical series:
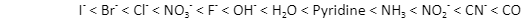
The ligands lying to the left of this series are weak field ligands and the ones lying to the right are strong field ligands.
CFT in Tetrahedral Complexes
The splitting here is opposite to how it happens in octahedral complexes. dx2-y2 and dz2 form a set of orbitals that are lower in energy. dxy, dyz, and dxz form a t2 set of orbitals that is higher in energy. The energy gap between the two is lower than that in octahedral complexes.
Ligand to Metal Charge Transfer (LMCT)
Sometimes, the colour of complexes appears even when there are no electrons in the d-orbitals of the metal. This happens because of the transfer of electrons from ligands to the empty orbital of the metal. It is observed in the case of complexes like Mn2O7- (intensely violet coloured), CrO42- (intensely orange coloured), etc.
Conclusion
We discussed why we could see colours in real life in this colour of complex notes. In coordination compounds, colour arises because of d-d electronic transitions. This can be explained by the crystal field theory. To understand the colour of complexes meaning, getting a hold on CFT is crucial. CFT happens differently in octahedral and tetrahedral complexes. Understanding the spectrochemical series of ligands is important to understand the differences in the colour of compounds. LMCT can give colours in compounds where d-d electronic transitions are not possible.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





