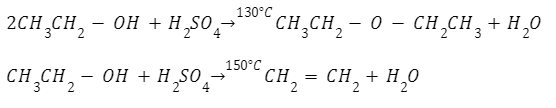It starts with Williamson synthesis, a crucial method for preparing unsymmetrical and symmetrical ethers. Ethers are organic compounds that contain Oxygen between two alkyl groups. That results in the formula R-O-R’ in which R is the alkyl group, and O is Oxygen. Ethers are also known as alkoxy alkanes. They are widely used in perfumes, oils, waxes and dye. In this reaction, an alkyl halide reacts with sodium alkoxide. Ethers with substituted alkyl groups can also be made by this method.
Dehydration of alcohols to make ethers
To successfully make ether by dehydrating alcohol, it is essential to have the right temperature. An SN2 reaction of alcohol conjugate acid produces ether at 110° to 130°C, and at the temperature of adobe 150°C, an E2 elimination occurs.
In the above reaction, the temperature is maintained at around 413K, and alcohol is in excess. If the reaction of both these procedures is not followed accurately, then the alcohol will undergo dehydration producing alkene.
The main product will be ethoxyethane when ethanol is dehydrated to ether in the presence of sulfuric acid at a temperature of 433K. It will not be possible to get ethers by the dehydration of secondary and tertiary alcohols, as alkenes are formed quickly in this reaction.
The above-given reaction can not be used to produce unsymmetrical ethers because a mixture of products is to be obtained. You can use the alkoxymercuration to produce unsymmetrical ethers.
The above-given reaction can not be used to produce unsymmetrical ethers because a mixture of products is to be obtained. You can use the alkoxymercuration to make unsymmetrical ethers.
Nomenclature of ethers
The common names of ether can be given by referring to the two groups connected to the ether oxygen. For example,
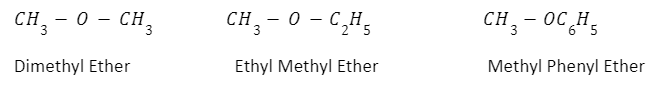
IUPAC names such as alkyl alkanes, alkoxy alkanes and alkyl arenes. In this, the longest chain is the root word and the parent chain.
Chemical properties of ether
Ethers are colourless or transparent fluids with a fruity odour and are highly flammable. Ethers are also soluble in water because of the H-bonding and Hydrophobic alkyl groups. Nevertheless, they lack hydrogen bonding, and hence the boiling point of ether is much less than other alcohols. Diethyl ethers can also be used as anaesthetic agents. The movements of C-O bonds do not cancel out as ethers have a bond angle of about 110°, and hence they have very weak polarity.
Physical properties of ether
Because hydrogen bond formation of ethers with the water molecules can contain up to 3 atoms of carbon in water, the solubility decreases, which means the increase in hydrocarbon atoms reduces the formation of H-bond. They have the formula R-O-R’ and contain an sp3 hybridised oxygen between alkyl groups. The lone pair electrons on Oxygen in aryl ethers are formed by the union of two compounds with the aromatic ring, which changes the property of ether.
Cleavage of C-O bond in ethers
The cleavage of the C-O bond in ethers requires extreme conditions or changes with excess HX and high temperature. The reaction of dialkyl ethers with HX gives two molecules of alkyl halides.
R-O-R+HX→R-X+R-OH
Alkyl aryl ethers are cleaved at the alkyl oxygen bond due to the more stable aryl oxygen bond, and breaking this bond is tough. The order of reactivity is HI>HBr>HCl. The reaction condition: excess(conc.) HI or HBr and high temperature.
When we have ether, two different alkyl groups are also cleaved similarly.
R-O-R’+HX→R-X+R’-OH
The mechanics here is that the first step is the protonation of ether.
Ether gets protonated in an acidic medium, and then we have protonated ether.
Then the second step is where the SN2 reaction occurs, where nucleophilic attacks from the less hindered side result in the corresponding substitutional response. We can see that I- attracts a methyl group, and then we have a transition state where the living group is the alcohol and methyl iodide. Note that we have excess HI and high temperature, which results in another substitution reaction giving us another molecule of an alkyl iodide.
In this case, we will see what happens when one of the alkyl groups is tertiary; the halide resulting is also a tertiary halide.
![]()
In the second step, the SN1 mechanism takes place where carbocation is formed.
Conclusion
We have discussed asymmetrical and symmetrical ether. We learned that ethers are organic compounds containing Oxygen between two alkyl groups and discussed how to make ethers by dehydration of alcohols. It is crucial to have the right temperature during the process. The cleavage of C-O bonds is related to the reaction time, solvent and temperature. In the absence of high hydrogen gas pressure and organic solvents, the catalyst can efficiently catalyse the cleavage of C-O bonds in water as a solvent. The protonation of the carbonyl oxygen activates the carbonyl group toward the nucleophilic addition of the alcohol.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out