Bond cleavage or bond fission is the splitting of chemical bonds. Dissociation occurs when a molecule is broken down into two or more components. Depending on the nature of the process, bond cleavage can be classified into homolytic and heterolytic.
The energy of a sigma bond’s triplet and singlet excitations can identify whether it will follow a homolytic or heterolytic pathway. A metal-metal sigma bond is an exception because the excitation energy of the bond is so high that observation is impossible.
Bond cleavage types
- Homolytic fission
- Heterolytic fission
Homolytic fission
Homolysis generates free radicals in the reaction.
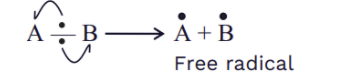
The factor which supports homolysis is zero or a small modification in electronegativity between A and B. i.e., μ = 0 , 𝚫EN =0 .Low electronegativity difference leads to formation of radical intermediate result due to homolytic bond breaking.Homolytic bond breaking takes place in gaseous phase or in the attendance of non polar solvents (CCl4, CS2 ).To assist pyrolysis, this form of bond breakage occurs under specific conditions, such as ultraviolet light, high temperatures, or high temperatures in the absence of oxygen. The amount of energy required for homolytic fission in a molecule is homolytic bond dissociation energy.
Heterolytic Bond Fission
In heterolysis, bond breaking in such a manner that bond electrons shift permanently to one fragment ,charged fragments produce (positive and negative) ions.
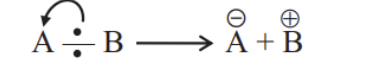
Bond polarity is the key reason behind heterolysis.The atom with a higher electronegativity tends to hold the bonding pair of electrons with itself. Heterolytic bond dissociation electricity is the quantity of strength essential for heterolytic fission in a molecule.
Drawing a curly curving arrow over the bond, pointing towards the most electronegative (A)atom involved in the bond formation, depicts heterolytic fission. The bonding pair of electrons with and H atoms is unequally distributed after heterolytic fission of the CH bond. The electronegativity of the C and H atoms is the deciding factor here. C has an electronegativity of 2.55, and H has an electronegativity of 2.20, according to Pauling. As a result, the electron bonding pair can be transferred to C, which has a higher electronegativity fee.
Reaction Intermediates
A response intermediate is a temporary species within a multi-step response mechanism. This is produced inside the previous step and fed on in a subsequent step to in the long run generate the final response product. Intermediate reactions are not unusual in the organic world; a prime example may be metabolite and nutrient metabolism.
Reactive, short lived, high energy, unstable species that are formed in the course of organic reactions are called reaction intermediates. Reaction intermediates are basically related to species formed in between reactions.
Carbocation ,Carbanion ,Free radical , Carbene and Nitrene are reaction intermediates.
For example : Assume A + B → C + D
Steps involved in the above reaction are
A + B → X*
X* → C + D
Where the chemical species X* is an intermediate.
Reaction Intermediates in Organic Chemistry
- Carbanion
In organic chemistry, there are various types of reaction intermediates. A carbanion is a natural chemistry reaction intermediate with a horrible fee on a carbon atom. Carbanions form when an organic component is treated with a completely robust base. An example is the reaction of butane with base.
The Carbanion is shaped when the bottom eliminates a hydrogen atom from the butane.
Carbanions are extraordinarily reactive, and they don’t last long after forming in a chemical reaction. They typically cross on to generate the reaction’s stop product by reacting with a fantastic species in the response. This makes experience because we are developing a negatively charged intermediate so that one can be pulled to a nice price.
- Free Radical
Another typical sort of chemical intermediate is free radicals. In unfastened radicals, there’s an unpaired electron. When a covalent link composed of electrons is broken, each atom takes one of the bond electrons. When a carbon-hydrogen bond in methane is broken, one of the bond’s electrons is transferred to carbon and the alternative to hydrogen. To reflect unfastened radicals, we utilise single dots at the atom where the novel is located.
3.Carbocation
Carbocation has a positive charge on carbon. Positively charged carbocation has only six electrons in the outermost shell. It has an incomplete octet. So it behaves like a Lewis acid. Since all electrons are in a paired state, carbocations are diamagnetic. Carbocations are sp2 hybridised. Carbocations are planar. Carbocations are formed in polar solvent. It stabilise by presence of electron donating group (EDG)
Conclusion
The breaking of a bond is known as bond cleavage. Because the electrons in a covalent bond are shared unequally among two atoms or similarly among the atoms, a bond can be damaged homolytically or heterolytically.
Covalent bonds, which include two atoms sharing electrons, are widely employed to create organic molecules.
The atoms that make up a covalent bond can break apart in two ways:
Homolytic fission
Heterolytic fission
Homolytic fission
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





