According to the energy profile diagrams in chemical kinetics, the transition states represent the maxima of energy. Whereas the reaction intermediates represent the minima of energy in the energy profile diagram. The reaction intermediates are somewhat stable species (as compared to transition states) that are stable enough to be observed, analysed and studied. These reaction intermediates are formed due to cleavage of the bond of carbon by either homolytic bond fission or heterolytic bond fission. Generally, whenever a bond breaks, a reaction intermediate is formed. Cleavage of the bond of the carbon atoms of a molecule lays the opportunity for new bonds to form.
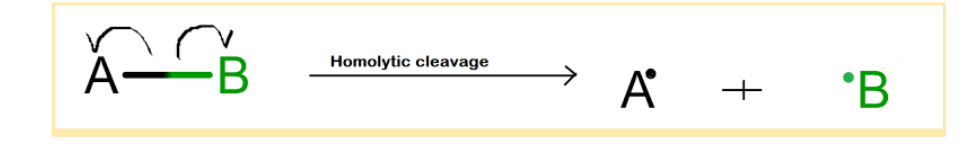
Homolytic cleavage
The definition of homolytic cleavage can be inferred from the name itself. ‘Homo’ means ‘same’. When a bond breaks by homolytic cleavage or homolytic bond fission, the electrons participating in covalent bond formation are equally distributed to both the constituent carbon atoms. This equal distribution of electrons to both atoms leads to the formation of uncharged species having seven electrons called radicals or free radicals. The carbon free radicals are reaction intermediates of many reactions such as halogenation in the presence of visible light, polymerization of alkenes, etc. Energy in the form of heat or light is required to initiate the process of cleavage of the bond of carbon. The homolytic cleavage of the bond of carbon is represented by a half-headed arrow; also known as a fish head arrow and each carbon atom in the product side is represented with a single unpaired electron. These arrows indicate the movement of electrons after the cleavage of the bond of carbon. Homolysis or homolytic bond cleavage most commonly happens in non-polar bonds.
![]() Heterolytic cleavage
Heterolytic cleavage
The definition of heterolytic cleavage can also be inferred from the name itself. ‘Hetero’ means ‘different’. When a bond breaks by heterolytic cleavage or heterolytic bond fission, the electrons participating in covalent bond formation are unequally distributed to both the constituent carbon atoms. This unequal distribution of electrons to both atoms leads to the formation of charged species called carbocations and carbanions. The carbocations are positively charged species having six electrons. The carbanions are negatively charged species having eight electrons. The carbocations are reaction intermediates of many reactions such as nucleophilic substitution reactions, addition reaction of alkenes, hydroboration and oxidation, many rearrangements, etc. The carbocations are reaction intermediates of reactions such as Decarboxylation, Formation of organometallic compounds, Addition of nucleophile to an alkene, etc. The heterolytic cleavage of the bond of carbon is represented by a full-headed arrow; also known as the double-barb arrow and the carbon atoms in the product side are represented as changed species with a positive and negative charge. The arrows indicate the movement of electrons after the cleavage of the bond of carbon.
![]() Ring opening
Ring opening
A reaction involving the opening of a ring usually takes place through heterolytic cleavage or heterolysis. The constituent atoms attached to the site of heterolytic fusion are not completely detached from the molecule i.e., the molecule remains as a single unit.
Cleavage of the bond of carbon examples
Homolytic cleavage of the bond of carbon examples-
Free radical addition to alkenes
In free radical addition to an alkene, a free radical is added to the alkene to form new carbon bonds and produce alkanes. In the given example, the peroxide linkage undergoes homolytic cleavage and generates two oxygen free radical molecules. This free radical reacts with an alkene and a carbon-free radical is produced in the course of the reaction. The newly produced carbon-free radical reacts with another molecule of alkene. In the final step of reaction no free radical remains.
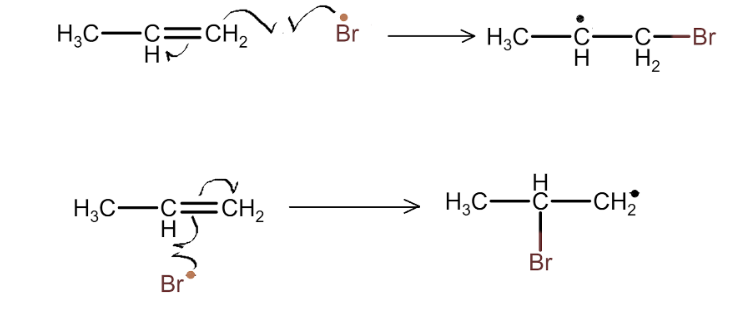
Halogenation
The unsaturated bond of alkene undergoes homolytic cleavage and reacts with bromine free radical. This initiates the free radical reaction of carbon. In the final step of reaction no free radical remains.
Homolytic cleavage of a bond of carbon examples-
Nucleophilic substitution reactions
The unimolecular nucleophilic substitution reactions or SN1 reactions are very slow reactions. The step in which the carbocation intermediate is formed is the rate-determining step of the reaction. The formation of the carbocation facilitates the inversion of the products. The formation of the carbocation takes place when the leaving group is lost from the site.
In the given reaction, the leaving group detaches from the carbon by heterolytic bond cleavage or heterolytic bond fission leaving behind a 6 electron highly reactive carbocation intermediate. Due to the presence of carbocation intermediate, inversion takes place and incoming nucleophile is attached on the opposite side.
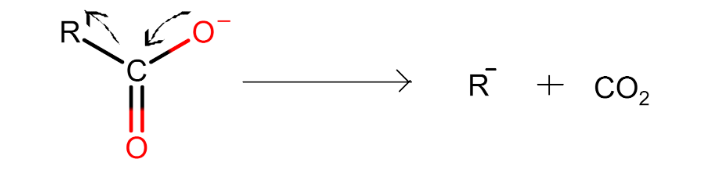
Decarboxylation
In decarboxylation, a carbanion intermediate is formed in the course of the reaction. The bond between the carbon of the alkyl group and the carbonyl group undergoes heterolytic cleavage or heterolytic bond fission. The alkyl group gains an electron and thus becomes a negatively charged species. The carbon of the carbonyl group forms a pi bond with oxygen to gain stability and produce carbon dioxide.
Conclusion
The cleavage of bonds of carbon importance is that without the cleavage of prior bonds new bonds can not be formed. To form better and stronger bonds old bonds must be broken to generate a more stable molecule. The cleavage of the bond of carbon either occurs via heterolytic bond fission or homolytic bond fission. The cleavage of bonds of carbon produces highly reactive species called reaction intermediates such as carbocations, carbanions and carbon-free radicals. These species react with other species or atoms to form new bonds. The cleavage of bonds of carbon and the formation of new bonds is the most basic principle of chemistry.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





 Heterolytic cleavage
Heterolytic cleavage Ring opening
Ring opening