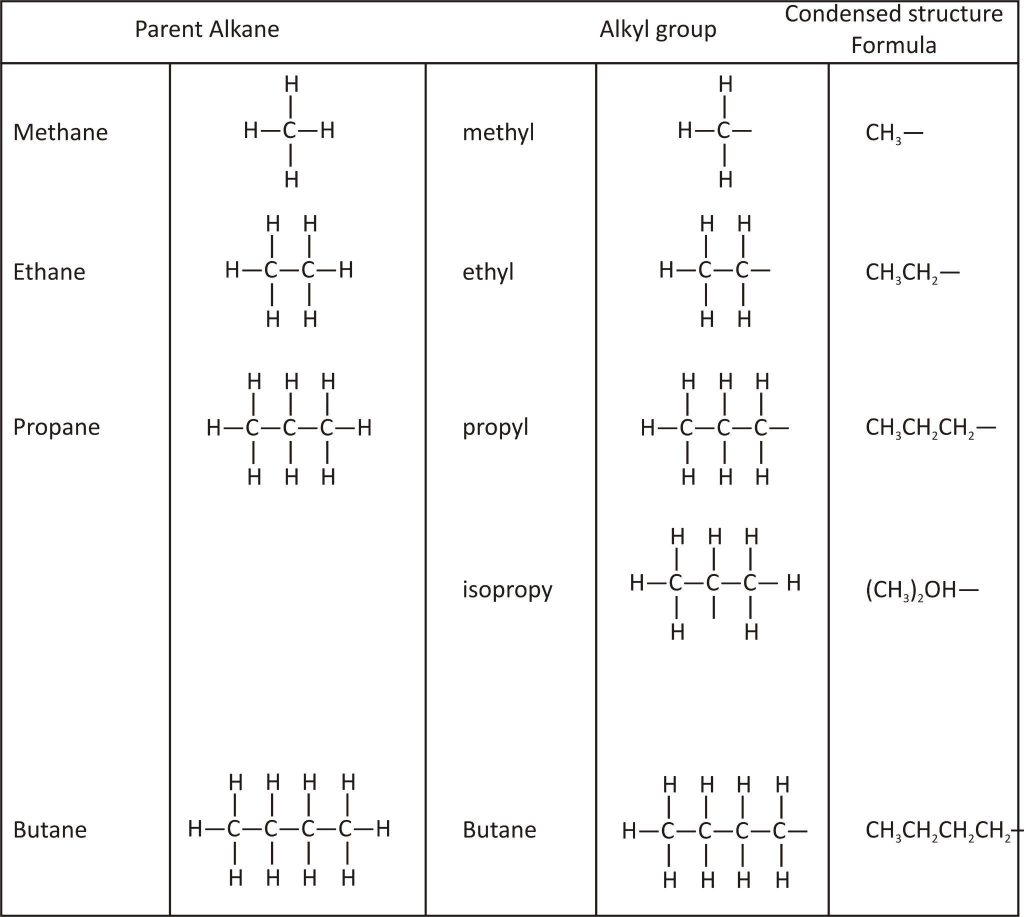
Classification
According to Berzelius, the compound which is made from living organisms is called an organic compound.
Vital force theory: (given by Berzelius) organic compounds can only be derived from living organisms and can’t be synthesized by the inorganic compounds in the laboratory. After some time
WOHLER Prove that organic compounds can also be made by Inorganic material
NH4CNO (lab method)
(Ammonium cyanate) (Urea)
(Inorganic compound) (organic compound)
Many organic compounds are made from inorganic material so finally vital force theory was collapsed.
Modern Definition of Organic compound
Organic Compound: Hydrocarbon and its derivatives are known as organic compounds.
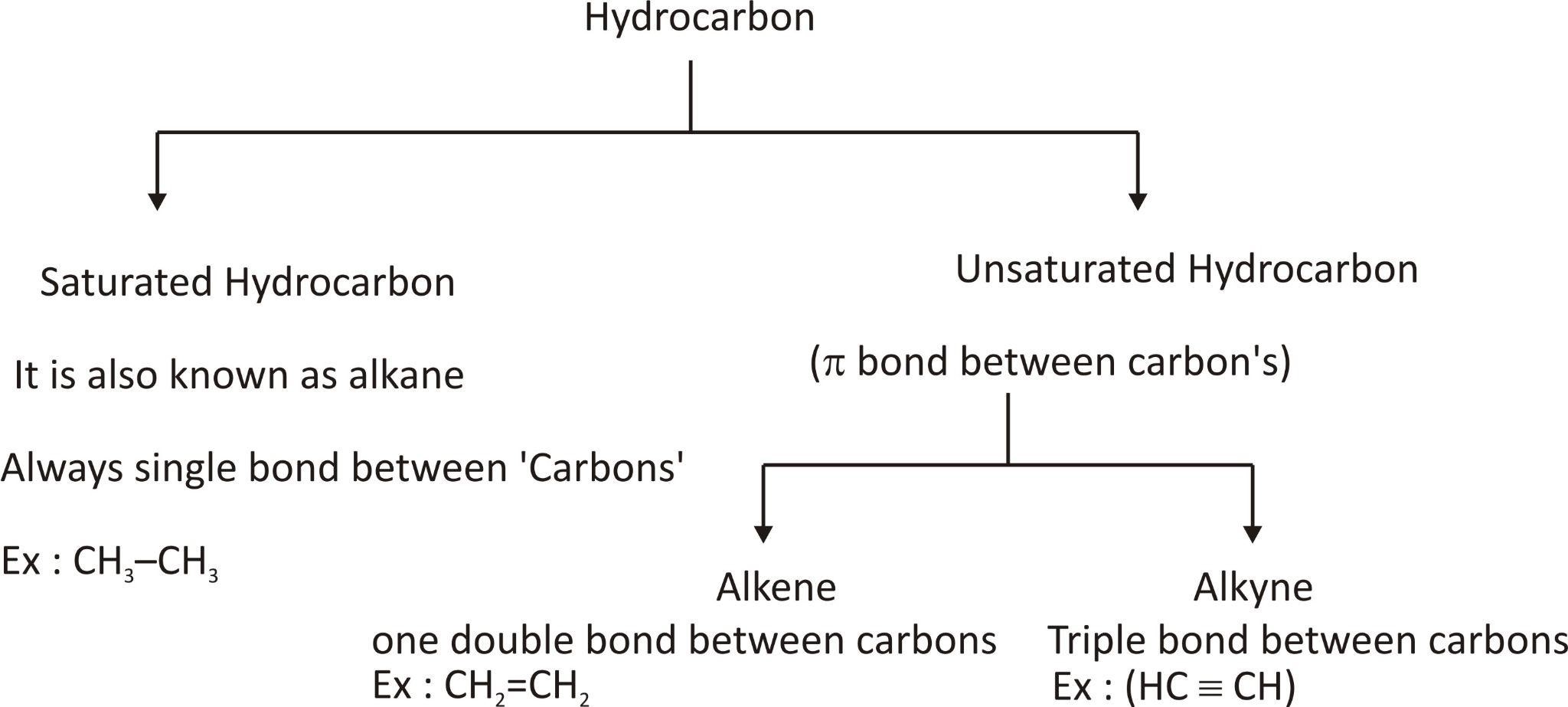
Hydrocarbon (H.C.): The compound which contains hydrogen (H) and carbon (C) or is made of H and C is called hydrocarbon.
CLASSIFICATION
IUPAC nomenclature
“IUPAC” is synonymous with “nomenclature” for scientists and chemistry students all around the world, particularly the nomenclature of organic chemistry. Using criteria set by the International Union of Pure and Applied Chemistry, generations of scientists have learned – sometimes reluctantly – to read and write systematic names for organic compounds. IUPAC names use prefixes, suffixes, numbers, and parenthesis to organize molecules: individually, by expressing the network of atoms and bonds that makes up an organic compound’s structure, and collectively, by placing each compound among the tens of millions of known organic chemical substances. Patent records, customs lists, and environmental regulatory databases all use IUPAC designations to carry this order out of chemical journals.
The IUPAC system of nomenclature was created to create a worldwide standard for naming substances in order to improve communication. The system’s purpose is to provide each structure a distinct and unambiguous name, as well as to associate each name with a distinct and unambiguous structure.
The IUPAC nomenclature system is based on designating the longest chain of carbons joined by single bonds in a molecule, whether in a continuous chain or a ring. Prefixes and suffixes are used to denote all deviations, whether multiple bonds or atoms other than carbon and hydrogen, according to a certain set of priorities.
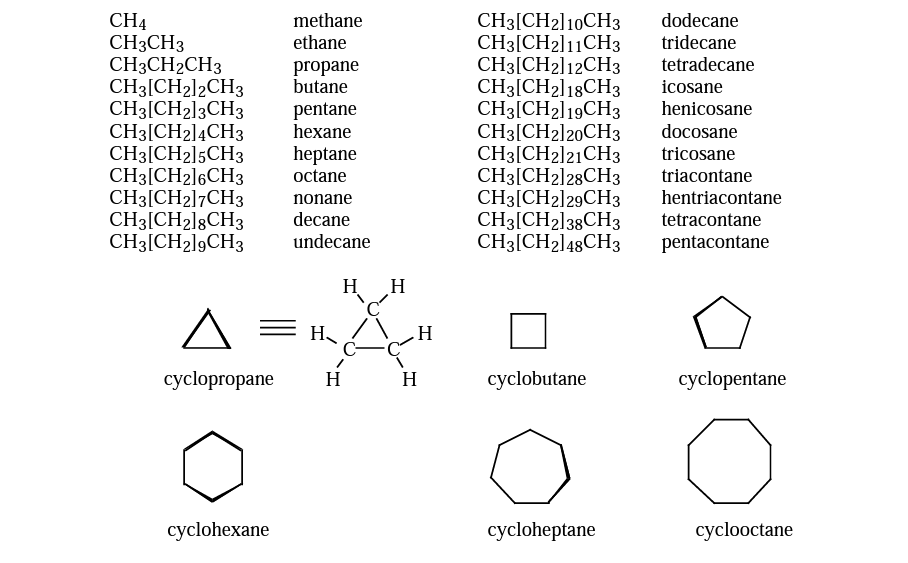
Alkanes belong to the saturated hydrocarbons family, which consists of molecules with only one bond connecting carbon and hydrogen. These molecules can be found in rings or in continuous chains (referred to as linear or acyclic) (called cyclic or acyclic). The root names for organic substances are alkanes and cycloalkanes. The number of carbons in the alkanes increases from five to ten starting with the five-carbon alkane.
The purpose of the IUPAC system of nomenclature is to establish an international standard of naming compounds to facilitate communication. The goal of the system is to give each structure a unique and unambiguous name and to correlate each name with a unique and unambiguous structure
IUPAC nomenclature is based on naming a molecule’s longest chain of carbons connected by single bonds, whether in a continuous chain or in a ring. All deviations, either multiple bonds or atoms other than carbon and hydrogen, are indicated by prefixes or suffixes according to a specific set of priorities.
Alkanes are the family of saturated hydrocarbons, that is, molecules containing carbon and hydrogen connected by single bonds only. These molecules can be in continuous chains (called linear or acyclic), or in rings (called cyclic or acyclic). The names of alkanes and cycloalkanes are the root names of organic compounds. Beginning with the five-carbon alkane, the number of carbons in the chain is indicated by the Greek or Latin prefix. Rings are designated by the prefix“cyclo”. (In the geometrical symbols for rings, each apex represents a carbon with the number of hydrogens required to fill its valence.)
- Name alkanes according to the LCC (longest continuous chain) of carbon atoms in the molecule (rather than the total number of carbon atoms). This LCC, considered the parent chain, determines the base name, to which we add the suffix -ane to indicate that the molecule is an alkane.
- If the hydrocarbon is branched, number the carbon atoms of the LCC. Numbers are assigned in the direction that gives the lowest numbers to the carbon atoms with attached substituents. Hyphens are used to separate numbers from the names of substituents; commas separate numbers from each other. (The LCC need not be written in a straight line; for example, the LCC in the following has five carbon atoms.)
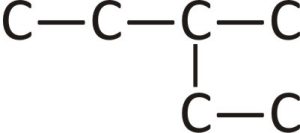
- Place the names of the substituent groups in alphabetical order before the name of the parent compound. If the same alkyl group appears more than once, the numbers of all the carbon atoms to which it is attached are expressed. If the same group appears more than once on the same carbon atom, the number of that carbon atom is repeated as many times as the group appears. Moreover, the number of identical groups is indicated by the Greek prefixes di-, tri-, tetra-, and so on. These prefixes are not considered in determining the alphabetical order of the substituents. For example, ethyl is listed before dimethyl; the di- is simply ignored. The last alkyl group named is prefixed to the name of the parent alkane to form one word.
When these rules are followed, every unique compound receives its own exclusive name. The rules enable us to not only name a compound from a given structure but also draw a structure from a given name. The best way to learn how to use the IUPAC system is to put it to work, not just memorize the rules. It’s easier than it looks.
Isomerism-
Isomerism is the phenomenon in which more than one compound has the same chemical formula but different chemical structures.
Chemical compounds that have an identical chemical formula but differ in properties and the arrangement of atoms in the molecule are called isomers. Therefore, the compounds that exhibit isomerism are known as isomers.
The word “isomer” is derived from the Greek words “isos” and “meros”, which mean “equal parts”. This term was coined by the Swedish chemist Jacob Berzelius in the year 1830.
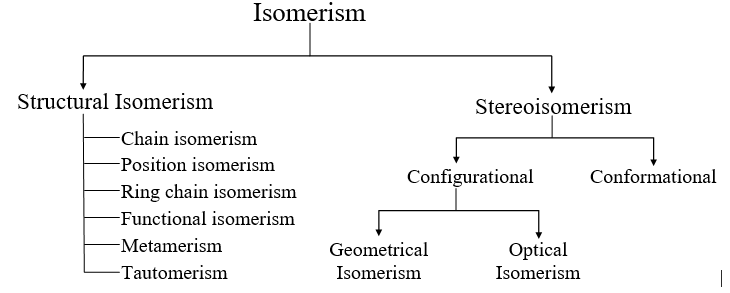
Isomerism Types
There are two primary types of isomerism, which can be further categorized into different subtypes. These primary types are Structural Isomerism and Stereoisomerism. The classification of different types of isomers is illustrated below.
Classification of Isomerism
Conclusion
The IUPAC system of nomenclature was created to create a worldwide standard for naming substances in order to improve communication. The goal of the system is to give each structure a unique and unambiguous name and to correlate each name with a unique and unambiguous structure.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out