A buffer solution is a solution that is added when the pH of the solution should be maintained even after adding small amounts of acid or base to that solution. In simple terms, we can call it a pH controller.They are basically an aqueous solution of a mixture of a weak acid and its salt or a weak base and its salt. Buffer solutions are mainly used in chemical reactions where the pH should be constant. For example, many biological reactions take place at a certain pH level only as the enzyme activation requires certain conditions for the reaction to take place.
Acidic buffer solution
It is a mixture of weak acid, its particular salt containing the conjugate base.
Example : CH3COOH + CH3COONa.
The pH of the acidic buffer should be less than 7 which represents their acidic nature.
When the ratio of acid to salt is changed, the pH can be changed accordingly.
Basic buffer solution
It is a mixture of a weak base and its particular salt-containing conjugate acid.
Example : NH4OH + NH4Cl.
The pH of the basic buffer should be higher than 7 which represents their basic nature.
The working mechanism of buffer solutions
The basic need of using a buffer solution in a chemical reaction is that it would control the pH even when acid or base or salt required for the reaction to take place is added in small amounts.
Eventually, we need a solution that contains the substance that will remove hydrogen ion [H+] or a hydroxyl ion [OH-] that might be added during a chemical reaction on the basis of acid or base added respectively, which helps in controlling the pH.
The steps utilised by acidic or basic buffers differ in achieving this.
By taking the example of acetic acid and sodium acetate, we can understand the mechanism better.
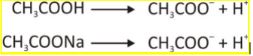
At this point, the solution has
- numerous un- ionised acetic acid
- Numerous Acetate ion
- free H + ions making the solution acidic.
Adding an acid:
Upon adding an acid, the H+ ions increase; therefore, the CH3COO– Ion combines with H+ ions to form acetic acid, which is a weak acid. Due to this, the equilibrium shifts to the right therefore, the pH of the buffer solution remains unchanged.
Adding a Base :
When a base is added to the buffer solution, the OH– ions add up with H+ to form a water molecule; it continues until all the h + ions are utilized. As a result, the equilibrium shifts towards the right and the pH value Rises slightly.
Working of a basic buffer is almost the same as the addition of an acid like HCl, the H+ ions combined with OH– form water.
The equilibrium shifts towards the right till all the H+ ions are used up. The pH of the buffer is restored on the addition of a base like NaOH, OH– combines with NH4+ ions to form unreacted Ammonium hydroxide. Thus, pH is maintained at constant.
Calculations of pH of a buffer solution:
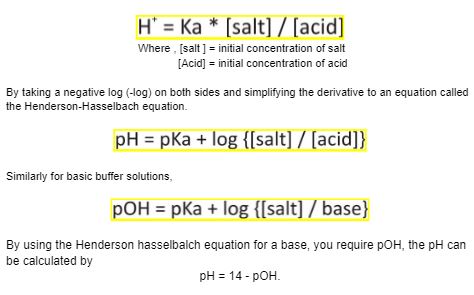
We can calculate the pH of an acidic buffer using the dissociation constant Ka
To calculate Ka we use the concentration of the acid and the salt used.
In any given weak acid, there is always an equilibrium between the un-ionized acid and its dissociated ion.
As weak acids only partially dissociate, they are suppressed by the addition of salt [Na+ A–] which gives more A- ions when the associated property is called the common Ion effect.
As a result, the equilibrium concentration of the unionized weak acid reaches nearly to its initial concentration. The equilibrium constant of the A– ion reaches almost equal to the initial concentration of the salt added.
Common ion effect:
The degree of dissociation of weak electrolytes is suppressed because of the common ions present in the solution. This is referred to as the common Ion effect.
For example, when Sodium Acetate and acetic acid are dissolved in the same solution, the dissociation of Sodium Acetate is higher than the dissociation of Acetic Acid as it is a weak acid and Sodium Acetate is a strong electrolyte.
Taking into consideration le chatelier’s principle, addition of Acetate ions from the Sodium Acetate will suppress the ionisation of Acetic Acid completely and shift its equilibrium towards left so the pH of the solution will increase. The presence of a conjugate base or acid of a particular acid or base acts as a limiting factor for ionization.
Therefore the common Ion effect plays an important role in the mechanism of how a buffer solution works.
Buffer capacity:
If the number of moles of many moles of a strong acid or base, which when added to 1 litre of a buffer solution, raises the pH by 1 unit.
the higher the capacity, the higher amount of acid or base could be added without changing the pH significantly.
by increasing the concentration of salt acid or base, the capacity of a particular buffer can be increased.
In conditions where,
the concentration of acid = concentration of salt
the concentration of base = concentration of salt
Then, pka = pH.
To prepare a buffer solution with a high capacity, always choose a weak acid or a base with pka value nearest to the pH value of the buffer solution required.
Conclusion:
Buffers are used in both real lives and in the laboratory. As we know, many biochemical reactions in a body take place only at a certain level of PH if this pH changes, high or low reaction stops.
Buffer solutions are used in biochemical assays where we find out the enzyme activities.
Buffer solutions are also used in dyes, Textile, and brewing factories.
The skin products which we use also need a perfect pH as it is used on the skin, and the buffer solutions are mainly used.
The above mentioned are a few of the applications of buffer solutions.
This particular Article highlights different types of buffer solutions, how they work, how they are prepared and how we can calculate the pH of these buffer solutions.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





