The Bronsted-Lowry acid-base theory (called Bronsted Lowry theory) classifies acids and bases according to whether they take or contribute protons or hydrogen ions. A Bronsted-Lowry foundation should have at most one lone pair of electrons that take a proton and create a new connection with that as well. An acid and a base interact with one another, allowing the acid to produce its conjugate base and also the base to generate its conjugate acid by swapping a proton, according to the above idea. In 1923, Johannes Nicolaus Brnsted & Thomas Martin Lowry separately presented the hypothesis. According to the Brasted-Lowry definition, an acid-base reaction would be any process where a proton is transported out of acid to a base.
About the Theory
Concerning the Hypothesis As per the Bronsted Lowry scheme, a component only behaves like acid in a base, and a component solely functions as just a base inside the presence of acid. Furthermore, whenever an acidic material loses a proton, it forms an acidic environment, the conjugate acid-base, but when a basic substance gains a proton, it generates an acid called the base conjugate acid. As a result, the interaction between being an acidic material, such as hydrochloric acid, as well as a basic substance, such as ammonia, may be described as follows:

The ammonium ion (NH4+) is the acid correspondent of the basic ammonia, while the chloride ion (Cl–) is the base correspondent of hydrochloric acid in the formula above.
The Bronsted–Lowry hypothesis further broadens the definition of acids and bases to include neutral molecules (such as alkali metal hydroxides and nitric, sulfuric, and acetic acids) and atoms and molecules with opposite electrical charges (called cations and anions). Acids include hydroxide ions, ammonium ions, and various saturated metal cations. Bases include phosphorus, acetate, sulphide, carbonate, and halogen ions.
Acids and Bases Definitions
Acids are chemicals that dissolve in an aquatic process to make H+ (hydrogen ions), whereas bases are materials that dissolve inside an aquatic solution to form OH–, as per the Arrhenius hypothesis (hydroxide ions).
In 1923, physical scientists Thomas Martin Lowry of England & Johannes Nicolaus Bronsted of Denmark developed the Hypothesis that bears their initials. This Bronsted Lowry theory represents acids and bases regarding how they respond to one another, allowing for more generality. The equilibrium phrase is used to describe this concept.
Acid + base ⇌ conjugate acid + conjugate base.
This equation may be represented symbolically using a HA acid as follows:

So because the reaction occurs through both forward and backward directions, the balance symbol (⇌) could be employed. The HA acid can lose a proton to become its conjugate base, A–. B, the base, may receive a proton and convert to HB+, the conjugate acid. Since most acid-base interactions are rapid, the constituents of the interaction are generally in new balance with one another.
Aqueous Substances
Assume the acid-base reaction shown below:
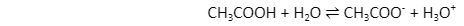
Acetic acid (CH3COOH) is acid because it transfers a proton onto water (H2O), thus forming an acetate ion (CH3COO–), being its conjugate base. Since it receives a proton from CH3COOH and converts it to its conjugate acid, the hydronium ion (H3O+), H2O is often used as a base.
An acid-base reverse reaction is an acid-base reaction that occurs in the acid’s conjugate base and also the base’s conjugate acid therein initial reaction. In the example above, acetate is used as the reverse reaction’s base, while the hydronium ion is used as the acid.
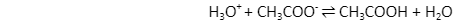
Unlike Arrhenius’ hypothesis, Bronsted-theory Lowry’s has the advantage of not requiring acid to dissolve.
The Lewis Acid-Base Theory is compared
- N. Lewis developed an alternative/substitutional explanation of acid-base interactions when Bronsted and Lowry released their theory. The electrical architecture is the basis for Lewis’ Hypothesis. A Lewis base is a molecule that can contribute a pair of electrons to a Lewis acid. On the other hand, A Lewis acid may take an electron pair. Lewis’ idea uses the electrical structure to describe the Bronsted–Lowry categorization.

In the above illustration, both of the conjugate basis, A–, and the nucleus, B, contain a lone pair of electrons, such as the proton, which would be a Lewis acid that may be transmitted between them.
Conclusion
In the perspective of proton transport among chemical species, the Bronsted-Lowry hypothesis analyses acid-base relations. Every species prepared to give a proton H+ is referred to as a Bronsted-Lowry acid. Any species capable of receiving a proton and requiring a lone pair of electrons to attach to the H+ is referred to as a Bronsted-Lowry base. Aqua is amphoteric, meaning it may serve as a Bronsted-Lowry acid and a Bronsted-Lowry base. Strong acids and bases ionise entirely during an aqueous solution, whereas weak acids and bases ionise just slightly.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





