Benzoylation is a chemical reaction in which a benzoyl group is instigated by removing H– that is attached to O or N or any aromatic ring.
It is a chemical reaction in which a benzoyl group is instigated by removing H– that is attached to O or N or any aromatic ring.
Hippuric acid is one example of a benzoylation reaction in which the -NH2 group is replaced by the benzoyl group and forms benzoyl glycine. Functional group benzoyl with the chemical formula C6H5CO–. There are many benzoyl compounds known. For example, benzoyl esters and amides are commonly used in organic chemistry. Esters can act as protecting agents in organic synthesis and can easily be removed by hydrolysis process via basic solution. A common example of benzoyl ketone is benzophenone.
Source of benzoyl group:
Benzoyl chloride is the commonly used chemical that favors the source of the benzoyl group. Which can be used in the preparation of benzoyl ketones, benzoyl esters, and for naturally occurring source of benzoyl group is thioester benzoyl -CoA.
Benzoylation in benzene contrasted with another benzene subordinate is less responsive. Since all hydrogen has a similar electronegativity. Benzoylation is a significant response for shaping bigger benzene moieties. For new medication planning purposes, many researchers utilize these strategies for amalgamation of new particles. In this response, bigger benzene moiety is likewise pertinent.
Benzoylation of Amines
The synthesis of amides from amines and acid chloride is also known as the Schotten Baumann reaction, named after chemist Carl Schotten Eugen Baumann. This is a type of condensation reaction.
Example of benzoylation of amines: Benzylamine reacts with acetyl chloride under desired conditions to form N- benzylacetamide as the final product. The required conditions for completion of the reaction are a two phase solvent system containing water and organic solvent. Acid is neutralized by the base present in the water phase, while starting material and product are left in the organic phase, often dichloromethane or diethyl ether.
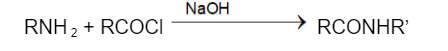
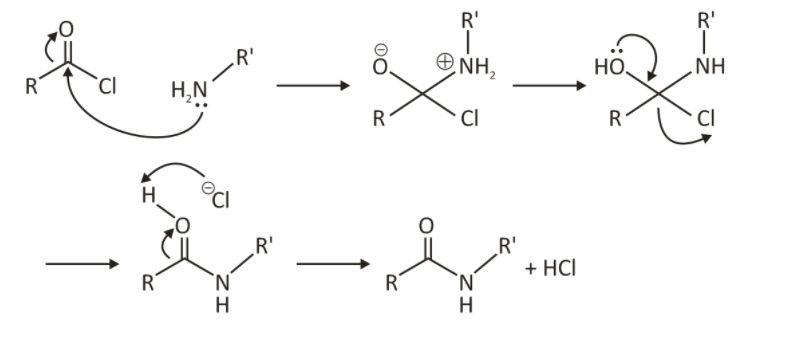
Mechanism:
Step 1: The protonated compound formed from the reaction between the acyl chloride and the amine. First, the nitrogen atom advances a lone pair of electrons towards developing a carbon-nitrogen bond.
The oxygen and nitrogen atoms having positive and negative charges, respectively, can be neutralized by exchanging a proton between them.
Step 2: The catalyst used in this reaction is a base that continues to absorb the acidic proton, which is shaped when oxygen endeavors to change a double bond with the carbonyl carbon.
Step 3: In the final step of the reaction, the expected amide atom is framed alongside hydrochloric atoms since the base catalyst has absorbed the acidic proton. The acid remains can be neutralized by the base also.
Benzoylation reagent
When it comes to carbohydrates diols, the most efficient and effective reagent was known as benzoyl-oxyma. It is highly crystalline and useful in the benzoylation of carbohydrate polyols. Benzoyl chloride is the commonly used chemical that favored the source of the benzoyl group. Which can be used in the preparation of benzoyl ketones, benzoyl esters, and for naturally occurring source of benzoyl group is thioester benzoyl -CoA.
Benzoylation of phenol
In this reaction, phenol reacts with benzoylation; the hydroxyl group replaces the hydrogen present in it with the benzoyl group to form phenyl benzoate.
Lately, it has been accounted for that an acetone arrangement of amines or amino acids, and benzoyl chloride is taken into brine, then sodium bicarbonate solution gives the benzoylated product.
Advantages of Benzoylation
There are, as a matter of fact, two significant benefits of benzoylation over acetylation, specifically: (a) First, for the most part, the benzoyl subordinates are got as crystalline solids having relatively higher melting point than the comparing acetyl subsidiaries, also having lower solubilities in a wide scope of solvents.
The base additionally neutralizes the hydrochloric corrosive, which is framed simultaneously, subsequently forestalling the further protonation of the amide product formed.
Typically, aqueous sodium hydroxide is utilized as the base catalyst. However, pyridine additionally can be used in this reaction.
By and large, the acyl chlorides are changed over into acylating agents of prevalent power when pyridine is utilized as the base catalyst.
Application of Benzoylation
The synthesis of capsaicin and phenethylamine is done by this method.
Using either acetyl chloride or acetic anhydride, acylation of Benzylamines can be done.
Peptides formed by the Fischer combination are one of the examples of Schotten Baumann reactions.
Conclusion
Benzoylation is a chemical reaction in which a benzoyl group is instigated by removing H- that is attached to O or N or any aromatic ring.
Hippuric acid is one example of a benzoylation reaction in which the -NH2 group is replaced by the benzoyl group and thus forms benzoyl glycine. A common example of benzoyl ketone is benzophenone.
Benzoyl chloride is the commonly used chemical that favored the source of the benzoyl group. The synthesis of capsaicin and phenethylamine is done by this method.
Peptides formed by the Fischer combination are one of the examples of Schotten Baumann reactions. There are, as a matter of fact, advantages of benzoylation over acetylation, specifically, for the most part, the benzoyl subordinates are gotten as crystalline solids having relatively higher melting point than the comparing acetyl subsidiaries, also, having lower solubilities in a wide scope of solvents. A few ideas utilized in the Schotten Baumann response are additionally utilized in the Fischer combination of peptides.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





