Anilineis an organic compound, and the formula for this is C₆H₅NH₂. Aniline is a simple aromatic anime that belongs to the phenyl group and an amino group. In chemistry, this is a significant compound that will effectively understand chemical compounds and their structure for different chemical bonding. This is a study material, which will provide notes on aniline structure reaction using different study material notes on aniline.
Aniline Structure
Aniline is a flammable liquid substance. It has an unpleasant odour with water-soluble properties. It can be both colourless and brown. It can feel oily when it is touched. Aniline also belongs to the phenyl group and has a chemical formula of C₆H₅NH₂ and C₆H₇N. The structure of aniline contains 6 carbon, 7 hydrogen, and 1 nitrogen atoms. Aniline is also classified as an organic compound because of the carbon in it.
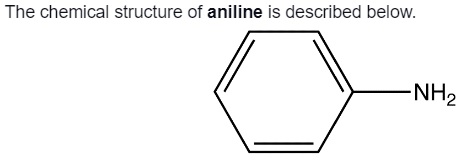
In the structure mentioned above, the blue box is the phenyl group. It is attached with the amino group, which is the red box. The phenyl group is a hexagon cycle that has alternative double bonds. The amino group contains hydrogen and nitrogen atoms in it. The chemical structure of aniline belongs to a phenyl group, and hence, we can call it aromatic. The chemical structure can be drawn in different ways to describe the chemical compound of aniline.
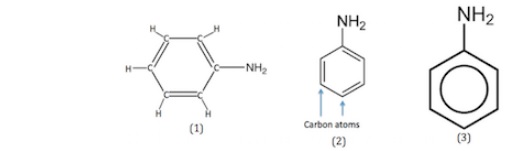
In the first structure, it is shown that atoms of hydrogen, nitrogen, and carbon are presented in this structure. In the second structure, hydrogen and carbon atoms are not shown. However, the structure is presented as a hexagon with double bonds. Finally, in the third structure, the alternating double bonds can be shown in a single ring of the circle.
Aniline Reaction
Oxidation
Aniline reaction has been investigated heavily in the matter of oxidation of aniline. The reaction with alkaline can create azobenzene, where arsenic acid can be produced via aniline of violet colouring. Chromic acid can convert into a Quinone. The presence of chlorate in metal salt can convert it into aniline black. Reaction with potassium chlorate and hydrochloric acid can create chlorine. Varieties of polyaniline can be made with oxidation using persulfate. The polymers contain rich redox and acid-base properties.
Nitrobenzene can be produced with oxidation in a neutral solution using potassium permanganate.
Hypochlorous acid can produce 4 Aminophenol and Para amino diphenylamine in the oxidation process.
Reaction with carbon
Aniline can have an electrophilic substitution reaction with carbon atoms like phenols. It enhances the electron-donating ability in the ring and has high reactivity of reflection as an enamine. The sulfuric acid and Aniline reaction can produce sulfanilic acid at 180 degrees centigrade. Bromine water is added to aniline. It is then decolourized, and a white precipitate of 2, 4, 6 tribromoaniline can be formed. Aniline can also react at room temperature with bromine in water.
Reaction with nitrogen
Aniline has a weak base. Aromatic aniline has a much weak structure than aliphatic amines. Aniline reacts with strong acids to form anilinium ions. The airline is attributed to a combination of inductive effects from more electronegative carbon and resonance. The lone pair of nitrogen can be delocalized in the benzene ring system.
Uses of Aniline
Anilines are used in preparing methylenedianilinel and related compounds with formaldehyde. It also uses rubber and processes chemicals, dyes, pigments, and many more. These study material notes on aniline will provide information about the various usages of aniline in different aspects.
Synthetic dye
In producing the first commercial synthetic dye, William Henry Perkin discovers quinine ad safranin, induline, and fuchsin. At the same time, mauveine was also discovered. At that time, aniline was very expensive, but it also created a revolution in the dye industry. As a result, the largest synthetic dye industry builds aniline dyes, and the first dye was named aniline yellow.
Medicine use
In the late 19th century, analgesic drugs were made of aniline. However, it has cardiac side effects, often beat with caffeine. During the beginning of the 20th century, chemotherapy as a magical revolution approached medicine while modifying the synthetic dye. It is widely used to prepare analgesics, antipyretics, vitamins and anti-allergic medicines. In 1932, Bart introduced the first usage of dyes in medical applications.
Rocket fuel
In rocket fuel, some early American rockets used aniline in the mixture. A mixture of aniline and furfuryl alcohol is used as a fuel along with oxidizer and nitric acid. The hypergolic, fuel and oxidation contact combination is used for a sorted period. Aniline was replaced by hydrazine later on.
Conclusion
Aniline is a toxic compound to inhale or infuse, and the use of aniline need many precautions. The early usage of aniline is for treating bladder cancer, but this is not effective now. Napthylamines are more effective now. Many methods are included in the dilution of anilines, and this aniline study material is presented to provide brief ideas on the aniline compound.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





