Anilines are a class of organic molecules known as amino benzene or phenyl amines in organic chemistry. A colouring chemical called aniline, widely used in the textile industry, is most likely the cause of colour on your blue jeans. Aromatic amines are a class of compounds that are known to be harmful. The characteristics of aromatic compounds may be found in these compounds used in various industrial settings.
Aniline meaning
Aniline is a Portuguese word consisting of ’’ Anil ’’ and ‘’the indigo shrub’’ and ‘’ine’’, indicating ‘’derived substance’’. Aniline was initially discovered in 1826 when indigo was subjected to a difficult distillation process.
What is Aniline?
Aniline is the simplest aromatic amine, comprising a phenyl group linked to an amino group. C6H5NH2 is the formula for aniline. It is a commercial chemical with great industrial importance and a diverse starting material for complex chemical processing. It possesses the distinct stench of rotting fish, as do the majority of volatile amines. It burns with a smoky flame, typical of aromatic chemicals, and it ignites easily.
Structure of Aniline
Aniline consists of six carbon atoms, seven hydrogen atoms, and one nitrogen atom, thus having the chemical formula of C6H7N or C6H5NH2. It is also an aromatic amine because it includes one amino group.
The hybridisation of nitrogen in aniline lies between sp3 and sp2 as it is pyramidal shaped. The nitrogen is regarded as having a strong p character. The lone pair is present in conjugation with the aryl substituent.
Physical properties of Aniline
- When exposed to the atmosphere and light, this organic molecule will darken.
- An anilinium ion C6H5-NH3+ is formed when it reacts with strong acids.
- When the substance is breathed via the air or absorbed through the skin, it is considered poisonous because it releases nitrogen oxides into the atmosphere, damaging the surrounding environment.
- Aniline has a boiling point of 184.13 degrees Celsius and a melting point of approximately -6.3 degrees Celsius.
- The distinctive smell of the substance distinguishes aniline.
Preparation of Aniline
The manufacturing of industrial aniline is a two-step process.
- An acidic solution of sulfuric acid is added to concentrated sulfuric acid to convert the benzene to nitrobenzene at temperatures between 50 and 60 degrees Celsius (122 and 140 degrees Fahrenheit).
- The nitrobenzene is then synthesised in the presence of metal catalysts (usually at 200–300 °C).
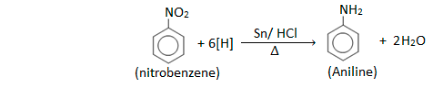
Chemical Reactions of Aniline
Aniline shows a variety of chemical reactions as mentioned below:
Basicity
The existence of a lone pair of electrons on the nitrogen atom makes it a basic element in nature. Because anilines are weaker bases, when they interact with stronger acids, anilinium ions are produced.
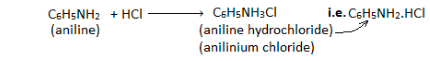
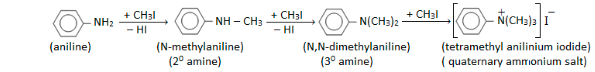
Alkylation
In alkylation reactions, aniline reacts with alkyl halides to form a combination of secondary, tertiary, and quaternary ammonium compounds.
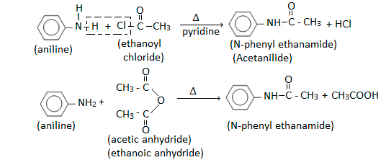
Acylation
The acylation process is characterised by anilines that prefer to react rapidly with carboxylic acids and create amides.
Diazotisation
Diazonium salts are produced when nitrous acid reacts with aniline or ring-substituted derivatives. To transform the amine group into one of these intermediates, Sandmeyer reactions may be used. Diazonium salt may be mixed with sodium nitrate (NaNO2) and phenol (benzene) to produce benzene-azo phenol, a colourant.
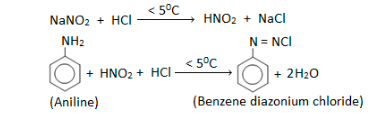
Oxidation reactions
Carbon-nitrogen bonds are often formed during the oxidation process of aniline compounds.
Carbylamine Reaction
In the presence of alcoholic KOH, chloroform is heated with a primary amine and then cooled to generate an objectionable odour of carbylamines. Specifically, the carbylamine reaction is the name given to this reaction.
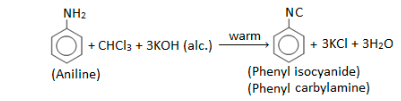
Other reactions like the hydrogenation and Wohl-Aue reactions are also there.
Uses of Aniline
The following are some examples of the many different ways aniline are put to use in science and everyday life:
- This chemical is utilised to produce pharmaceuticals such as paracetamol, acetaminophen, and Tylenol.
- This chemical is used as a pesticide and a fungicide in agriculture.
- It is used in creating polyurethane, which is subsequently utilised in the manufacturing of plastics.
- It is also used in the rubber industry in vulcanisation.
- Vitamins, analgesics, antipyretics, and antiallergics are among the many products made from aniline.
- It is used to manufacture dyes, perfumes, and resins.
Conclusion
One of the most powerful organic chemicals in the world, Aniline, is produced every year in millions of tonnes. The nitration of benzene and the hydrogenation of the nitrobenzene formed in the process of commercial manufacturing of aniline are the two most important chemical processes in the creation of aniline. It features a ring-like structure that is reminiscent of a hexagonal shape. Aniline is a molecule with a closed chain structure; it is not a linear molecule. In its liquid form, aniline is a highly explosive chemical compound with a strong, unpleasant odour that is only minimally soluble in water. This substance is colourless to brown in appearance and feels greasy to the touch. The majority of its uses are in polymer chemistry, but it is also used in the manufacturing of rubber chemicals, dyes, medicines, and other chemical specialties.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





