Introduction
This topic educates the learners on Sulphur and its allotropic forms. Here, we will discuss Allotrope of Sulphur, Allotropic forms of Sulphur, and how these Sulphur Allotropic forms are prepared. This article will also help you to understand the basics of Sulfur, the Allotrope of Sulphur, and which is the most stable allotropic form of Sulphur.
Inorganic chemistry is a part of Chemistry for IIT Jee mains exam preparation that requires a lot of mugging. Sulphur is an important part of the periodic table that falls under the topic Allotropic forms of Sulphur.
What is Sulphur?
In the periodic table, sulphur can be found in group 16. The earth’s crust consists of sulphur 0.17 % of the total Earth’s Crust. It is non-metal and can be obtained as a by-product after natural gas production. When sulfur burns, toxic gas is created known as sulfur dioxide. Pure sulfur has no smell and sulfur compounds are called mercaptans.
What is the Allotrope of Sulphur?
Sulphur forms more than 30 Allotropes, which is more than any other element. We will discuss the three most important Allotropes of Sulphur. They are as follows:
- Rhombic Sulphur or α-sulphur
- Monoclinic Sulphur or β-sulphur
- Plastic Sulphur or γ-sulphur
What is the Allotrope of Sulphur?
The three Allotrope of Sulphur are described below:
What is Rhombic Sulphur or α-sulphur?
- The nature of the Rhombic Sulphur is Crystalline and its shape is Octahedral.
- We will get rhombic sulphur by heating the solution of roll sulphur in the CS2.
- Its colour is yellow and is the most common form of Sulphur.
- Rhombic Sulphur’s m.p(melting point) is 385.8K.
- Rhombic Sulphur’s specific gravity is 2.06.
- Rhombic Sulphur is not soluble in water but can dissolve in alcohol, ether, and benzene to some extent.
- Rhombic Sulphur is readily soluble in CS2.
- It is the most stable allotropic form of Sulphur at room temperature.
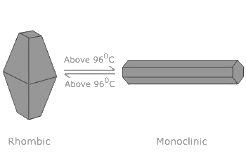
- When slowly heated to 96°C, it changes into β–sulphur or monoclinic Sulphur. But when cooled below 96°C, it returns to its rhombic form.
- It exists as S8 Molecules. The Sulphur atoms are arranged in a puckered ring.
How is it Prepared?
It is formulated by dissolving ground sulphur in CS2 at room temperature. The mixture is then purified. The filtrate is then kept in a small beaker insulated with a filter sheet. The CS2 will slowly evaporate away leaving behind large octahedral crystals of rhombic sulphur or α–sulfur.
What is Monoclinic Sulphur or β-sulphur?
- The monoclinic sulphur is stable only above 96℃
- The monoclinic Sulphur melting point is 393K.
- Monoclinic Sulphur specific gravity is 1.98.
- Monoclinic Sulphur exists in S8 ring molecules.
- It has a Crystalline Structure.
- It is in dull yellow and is soluble in CS2.
- α sulphur is stable below 369K and it becomes β-sulphur above that temperature while β-sulphur is stable above 369K and transforms above 369K in α sulphur. This is the reason the 369K temperature is known as the transition temperature.
How is it prepared?
The Monoclinic Sulphur is prepared after melting Rhombic sulphur in a dish and cooling it until the crust is formed. Two holes are made in the crust and the rest of the liquid is poured out. Then the crust is removed from the dish. Long Needle-shaped Crystals of Monoclinic Sulphur are formed on the lower side of the crust.
What is Plastic Sulphur or γ-sulphur?
- Plastic Sulphur is another allotrope of Sulphur.
- It is one of the amorphous allotropes of Sulphur
- Gamma Sulphur is a tough elastic substance.
- Plastic sulphur is soft and can be easily stretched.
- Plastic sulphur has no sharp melting point.
- 1.95g cm-3 is the specific gravity of plastic sulphur.
- It is insoluble in CS2.
- It is unstable at all temperatures.
 How is it prepared?
How is it prepared?
It is formed by unexpectedly chilling the molten sulphur (near its boiling point) by dropping it into cold water. Yellow rubbery ribbons of sulphur are constructed. They are very soft and can be stretched effortlessly. It is a dark brown rubber-like mass that can be squeezed between fingers, hence plastic.
What are the Important Uses of Sulphur?
Some of the important uses of Sulphur are as follows:
- Its compounds can be used to formulate specific kinds of fungus in the vines.
- For the formation of tetraoxosulphate(VI) acid sulphur is used as a common ingredient. It is also an essential use of Sulphur.
- In the making of Ca(HSO3)2 (calcium hydrogen Tetraoxosulphate (IV)) it is also useful.
- The sulphur compound is as useful in the paper manufacturing industry as wood pulp.
- It is a common and important ingredient in rubber vulcanization.
- It is used in dye manufacturing.
- Sulphur has important use in ointments.
- Sulphur can be used in the making of matchsticks, firecrackers, and Gunpowder.
Conclusion
Sulphur is one of the substances in the periodic table that is found in group 16. It has numerous allotropes of sulphur but three are the most important allotropes of sulphur. They are rhombic sulphur h monoclinic Sulphur and plastic sulphur. Rhombic sulphur is the most common allotropic form of Sulphur and is the most stable allotropic form of Sulphur at room temperature.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






