Adsorption isotherms are leveraged for research related to adsorption techniques and environmental protection. It is normally studied using a graph called adsorption isotherm. It reflects the amount of adsorbate present on the adsorbent in the form of a function with concentration or pressure at a constant temperature. The adsorbed quantity is normalised by adsorbent mass to compare different materials.
Adsorption of Isotherms
An overview of adsorption isotherms:
- It is used to link adsorption equilibrium data to characterise sorbent materials.
- It’s used in the development of industrial gas adsorption techniques.
- There are isotherms of gas-phase adsorption.
It is supplied for the design of industrial gas adsorption processes. The adsorbate’s partial pressure (gas, vapour) or concentration (liquid) in the fluid, as well as the adsorbent’s solute loading, which is defined as mass, mole, or volume of adsorbate per unit mass, mole, or volume of the adsorbent, are used to express the equilibrium.
Compared to vapour-liquid and liquid-liquid ratios, where theory is routinely employed to estimate phase distribution, there is no suitable theory for fluid-solid adsorption equilibria. Thus, experimental equilibrium data for a specific solute, or combinations of solutes or solvent, and a sample of the real solid adsorbent material of interest, are required, as explained in the overview of adsorption isotherms.
If the data are collected across a range of fluid concentrations at a constant temperature, a plot of solute loading on the adsorbent vs concentration or partial pressure in the fluid can be generated. This is an example of an adsorption isotherm diagram. We concentrated on adsorption in the gas phase. Adsorption in the liquid phase has its isotherms.
Isotherm of Freundlich Adsorption
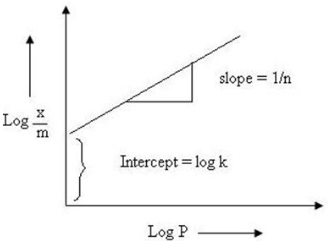
In 1909, a German chemist named Freundlich proposed an empirical relationship between the amount of gas adsorbed by a unit mass of solid adsorbent and pressure at a particular temperature. To express it, we use the following equation:
x/m = k.P1/n (n > 1)
where ‘x’ denotes the mass of the gas adsorbed on the adsorbent’s mass ‘m’. The variables ‘k’ and ‘n’ are determined by the adsorption type and the gas at a given temperature.
The mass of the gas adsorbed per gram of adsorbent is plotted versus pressure in the shape of a curve to highlight the relationship. Physical adsorption reduces with increasing temperature at a constant pressure. At high pressure, the curves achieve saturation. If you take the log of the above equation, you’ll get
log x/m = log k + 1/n log P
To check if the Freundlich isotherm is genuine, plot log x/m on the y-axis and log P on the x-axis. The Freundlich isotherm is legitimate if the plot shows a straight line; otherwise, it is not. The value of 1/n is given by the slope of the straight line, whereas the intercept gives the value of log k on the y-axis.
Limitations of the Freundlich Isotherm
The Freundlich isotherm only approximates the adsorption behaviour. Furthermore, because the value of 1/n might be anywhere between 0 and 1, the equation is only valid for a narrow range of pressures.
If 1/n = 0, x/m is equal, adsorption is pressure variable.
Adsorption is exactly proportional to pressure when 1/n = 1, x/m = k P
Both of the conditions above are supported by experimental results. The experimental isotherms always appear to approach saturation at high pressure. The Freundlich isotherm fails to explain this finding at high pressure since it does not account for it.
Two more isotherms followed the Freundlich isotherm: the Langmuir adsorption and BET adsorption isotherm. The Langmuir isotherm assumed monolayer adsorption in nature, whereas the BET isotherm assumed multilayer adsorption.
Isotherm of BET Adsorption
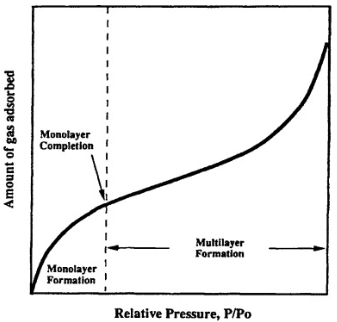
The theory explains the adsorption process of gas particles on a solid surface and forms the basis for a critical examination of the material’s specific surface area measurement.
Characteristics
- Only on well-defined adsorption active sides does adsorption take place.
- Adsorption is a multilayered process.
- Adsorption is a physical process.
- The adsorbate molecules in the uppermost layer are in equilibrium.
Conclusion
We have learned about Freundlich and Langmuir’s introduction to adsorption isotherm. Temperature effects on adsorption, adsorption from liquids, and certain adsorption applications. Some examples of adsorption uses are as follows:
- Poisonous gases are adsorbed to the surface of coal workers’ gas masks, preventing them from coming into touch with them.
- A vacuum is formed by adsorbing residues of air on charcoal and removing them from devices that are being evacuated.
- Silica gel pellets absorb moisture and control humidity in medications and new plastic bottles.
- Colour removal to obtain a clear liquid solution, cane juice is treated with animal charcoal to remove the colouring element.
- Catalysts are people who help things happen. As catalysts, appropriate materials are used to attract reactants to their surfaces, allowing the reaction to proceed more quickly and at a faster pace.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





